straumann® dental implant system one system â one instrument kit
straumann® dental implant system one system â one instrument kit
straumann® dental implant system one system â one instrument kit
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Straumann ® Dental<br />
<strong>implant</strong> <strong>system</strong><br />
ONE SYSTEM – ONE INSTRUMENT KIT –<br />
ALL INDICATIONS*
For all indications*!<br />
The Straumann ® Dental Implant System offers you and your patients<br />
efficient and esthetic <strong>implant</strong>-borne restorations for a wide range of indications,<br />
giving you the flexibility you need in your treatment.<br />
Single tooth missing<br />
Partially edentulous<br />
Fully edentulous<br />
*From single teeth to edentulous solutions
SIMPLICITY AND EFFiciency: SOFT TISSUE LEVEL SOLUTIONS<br />
Straumann ® Soft Tissue Level is <strong>one</strong> of the most<br />
widely used soft tissue <strong>implant</strong> lines. With 10-year<br />
long-term results for SLA ®1 , it is the ideal partner in<br />
your practice and the right choice for your patient<br />
treatment.<br />
Designed to save time and<br />
increase efficiency in your practice 2<br />
Soft Tissue Level <strong>implant</strong> aims at reducing chair time and<br />
minimizing treatment complexity<br />
Designed to provide integrated soft tissue management<br />
through built-in machined collar design<br />
High treatment success and patient satisfaction supported<br />
by strong scientific evidence 3<br />
Treatment workflow on soft tissue level IMPlant SolutionS<br />
Placement of an <strong>implant</strong> Healing cap Placement of a provisional<br />
abutment<br />
2
REDUCING TREATMENT compleXity<br />
Take advantage of the easy accessibility<br />
to the <strong>implant</strong> by working<br />
at soft tissue level<br />
Lean prosthetic portfolio supported by the <strong>implant</strong> design<br />
Abutment-level impression makes workflow efficient and<br />
number of patient visits can be minimized 4<br />
Simple impression taking and abutment placement due to<br />
the connection at soft tissue level<br />
Conventional impression taking Insertion of the abutment Crown in place<br />
3
ESTHETICS AND FLEXIBilty: BONE LEVEL IMPLANT SOLUTIONS<br />
The Straumann ® B<strong>one</strong> Level <strong>implant</strong> line provides<br />
you with additional flexibility to deliver an optimal<br />
esthetic solution to patients. B<strong>one</strong> Level <strong>implant</strong>s<br />
complement the Straumann ® Soft Tissue Level portfolio<br />
to offer you wide treatment flexibility and manifold<br />
options.<br />
Excellent esthetics due to<br />
b<strong>one</strong> preservation 5,6 and smart<br />
soft tissue management<br />
Stable crestal b<strong>one</strong> supporting esthetic outcome 5,6<br />
Exclusive self-guiding CrossFit ® connection designed for<br />
a precise fit and stability<br />
A wide range of treatment options with the convenience<br />
of a confident solution at b<strong>one</strong> level<br />
B<strong>one</strong> level <strong>implant</strong><br />
The precise and self-guiding<br />
CrossFit ® connection<br />
4
FOR A WIDE ranGE OF treatment optionS<br />
Designed for outstanding<br />
esthetic results and flexibility in<br />
restorative procedures<br />
Broad range of materials and restorative options<br />
designed for an optimal result in every treatment step<br />
Natural appearance through full ceramic restorations<br />
with customized or standard abutments<br />
Smart soft tissue management from start to finish with<br />
Consistent Emergence Profiles<br />
Consistent Emergence Profiles<br />
Consistently matching geometries throughout the entire<br />
work flow<br />
Eases the fabrication of temporary and final restorations<br />
Excellent esthetic results 5,6<br />
Healing abutment Temporary restoration Final restoration<br />
5
A LEADING SURFACE innoVation: SLActive ®<br />
MORE CONFIDENCE THROUGH<br />
HIGHER PREDictability 9,10,11<br />
SLActive ® designed to deliver:<br />
Higher security and faster osseointegration for every<br />
indication 9,10,12–17<br />
Reduced healing times from 6–8 weeks down to<br />
3– 4 weeks 11<br />
Increased treatment predictability in critical protocols 9<br />
For years, <strong>implant</strong>s with the SLA ® surface have delivered<br />
excellent results and high <strong>implant</strong> success<br />
rates. 7,8 Straumann followed this state-of-the-art<br />
development in <strong>implant</strong> dentistry with the SLActive ®<br />
surface, the next generation in surface technology.<br />
Promoting faster osseointegration and providing<br />
higher predictability, SLActive ® has become a central<br />
feature of the <strong>implant</strong> portfolio.<br />
6
DRIVING material innoVation: ROXOLID ®<br />
Roxolid ® , the new “DNA” of <strong>implant</strong> materials, is a<br />
titanium-zirconium alloy, specifically developed for<br />
<strong>dental</strong> <strong>implant</strong>ology. Roxolid ® is designed to widen<br />
your treatment options with small diameter <strong>implant</strong>s.<br />
Mechanical tests have demonstrated higher tensile<br />
ROXOLID ® THE NEW “DNA” OF<br />
IMPLANT materialS<br />
Confidence when placing small diameter <strong>implant</strong>s<br />
Flexibility of having more treatment options<br />
Designed to increase patients’ acceptance of <strong>implant</strong><br />
treatment<br />
and fatigue strength of Roxolid ® <strong>implant</strong>s compared<br />
to titanium grade 4 <strong>implant</strong>s. 18,19 In combination<br />
with the SLActive ® surface, Roxolid ® brings high<br />
strength and excellent osseointegration properties<br />
to the Straumann ® Dental Implant System portfolio.<br />
7
SIMPLICITY: ONE SYSTEM<br />
The proven Straumann ® Dental Implant System (SDIS)<br />
is a broad portfolio of Soft Tissue and B<strong>one</strong> Level<br />
<strong>implant</strong>s that allows you to deliver an optimal treatment<br />
to your patients.<br />
Implant portfolio<br />
The classic<br />
<strong>implant</strong> for<br />
<strong>one</strong>-stage<br />
surgery<br />
The <strong>implant</strong><br />
for flexible<br />
healing<br />
protocols<br />
The <strong>implant</strong> for<br />
immediate<br />
placement and<br />
superior primary<br />
stability<br />
The <strong>implant</strong><br />
for confidence<br />
and esthetics<br />
at b<strong>one</strong> level<br />
Most common<br />
clinical situations<br />
Straumann ®<br />
Standard<br />
Straumann ®<br />
Standard Plus<br />
Straumann ®<br />
Tapered Effect<br />
Straumann ®<br />
B<strong>one</strong> Level<br />
Anterior region •<br />
Posterior region • • • <br />
Small inter<strong>dental</strong> space (NN) •<br />
Thin mucosa biotype •<br />
Thick mucosa biotype • • • <br />
B<strong>one</strong> augmentation with<br />
simultaneous <strong>implant</strong> placement<br />
•<br />
Submerged healing •<br />
Non-submerged healing • • • <br />
Immediate <strong>implant</strong> placement in<br />
extraction socket<br />
• •<br />
Internal sinus lift • • <br />
• Highly recommended<br />
Recommended<br />
8<br />
Please note that the size limitations according to the instructions for use of each Straumann ® product apply and must be observed.<br />
Each Straumann ® product must be used in accordance with the instructions for use provided by Straumann. It is the practiti<strong>one</strong>r’s<br />
responsibility to use the device in accordance with these instructions for use and to determine if the device fits the individual patient<br />
situation.
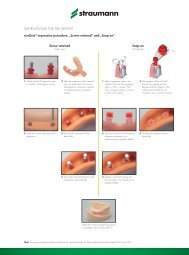
SIMPLICITY: ONE INSTRUMENT KIT<br />
For your convenience, the appropriate Straumann ®<br />
surgical <strong>instrument</strong> <strong>kit</strong> can be used for all <strong>implant</strong><br />
lines of the Straumann ® Dental Implant System, in<br />
both conventional and guided surgery.<br />
FOR EASE OF HANDling<br />
The easy-to-read user guide ensures a reliable<br />
working sequence through color-coded arrows and<br />
silic<strong>one</strong> sleeves<br />
The cassette can be packed to reflect the working<br />
procedure (which is performed either using the handpiece<br />
or manually with the ratchet)<br />
Clear illustrations and drill length stops confirm,<br />
at a glance, that the arrangement of <strong>instrument</strong>s, screws<br />
and healing caps is complete and correct<br />
Straumann ® Instrument Kit<br />
Straumann ® Surgical Cassette<br />
Straumann ® Guided Surgery Cassette<br />
9
PROSTHETIC OPTIONS COVERING A Variety OF INDicationS<br />
Choose between different materials and follow<br />
your preferred workflow! The Straumann ® prosthetics<br />
portfolio offers you the esthetics and flexibility<br />
you need in your practice.<br />
CHOOSE THE accurate PROSTHETIC OPTION<br />
Solid abutments are designed to facilitate the restoration<br />
of <strong>implant</strong>s<br />
Flexible and comprehensive synOcta ® portfolio offers the<br />
perfect restorative solution<br />
Easy to achieve esthetics with anatomic abutments<br />
Prosthetics portfolio<br />
Titanium<br />
Cementable Solid synOcta ®<br />
Cementable<br />
synOcta ®<br />
Angled<br />
Anatomic<br />
CADCAM<br />
Screw-retained<br />
•<br />
Single tooth<br />
Cemented • • • • <br />
Screw-retained<br />
•<br />
Partially edentulous<br />
Cemented • • • • <br />
Basic Solutions<br />
Advanced<br />
LOCATOR ® Bar Retentive<br />
anchor<br />
Individualized bar<br />
Removable<br />
overdentures<br />
• • • • <br />
Fully edentulous<br />
• Soft Tissue Level <strong>implant</strong>s<br />
Fixed overdentures<br />
B<strong>one</strong> Level <strong>implant</strong>s
Natural-looking esthetics with CADCAM abutments<br />
Flexible and comprehensive Multi-Base portfolio designed<br />
for perfectly fitting screw-retained multiple unit restorations<br />
Due to their standard designs, LOCATOR ® and bar<br />
comp<strong>one</strong>nts are ready to use in a variety of indications<br />
Gold Ceramics Cobalt-chrome<br />
synOcta ®<br />
1.5<br />
Multi-Base Straumann ® CARES ®<br />
Screw-retained bridge<br />
synOcta ®<br />
Gold<br />
Gold Anatomic IPS e.max ®<br />
Abutment<br />
CADCAM Straumann ® CARES ®<br />
Screw-retained bridge<br />
• • • <br />
• • <br />
• • •<br />
• • <br />
Solutions<br />
Premium Solutions<br />
Telescopic constructions synOcta ®<br />
1.5<br />
Multi-Base Gold Straumann ® CARES ®<br />
Screw-retained Dolder ® Bar<br />
Straumann ® CARES ®<br />
Screw-retained bridge<br />
• •<br />
• •
RELIABILITY: BONE CONTROL DESIGN<br />
B<strong>one</strong> Control Design is an <strong>implant</strong> design which<br />
has been applied consistently to the Straumann ®<br />
Dental Implant System. It is built on 5 key factors for<br />
b<strong>one</strong> preservation and thus provides a crucial foundation<br />
for esthetic results and long-term success.<br />
The Straumann ® Dental Implant System is your reliable<br />
partner in <strong>implant</strong> treatment, giving you simple<br />
and convenient solutions – for your success!<br />
Optimize crestal b<strong>one</strong> preservation<br />
1. Respecting the biological distance<br />
5<br />
4a. Microgap control Soft Tissue Level<br />
4b. Microgap control B<strong>one</strong> Level<br />
2. Optimal position of smooth and<br />
rough surface interface<br />
5. Implant surface osteoconductivity<br />
3. Biomechanical <strong>implant</strong> design<br />
12
eferences<br />
1<br />
Fischer K. ITI World Symposium. 2010 15–17 Apr; Geneva; Switzerland.<br />
2<br />
Applicable to simple cases according to the ITI rating.<br />
3<br />
Straumann ® SLA Scientific Evidence first edition (2011).<br />
4<br />
Applicable to simple cases according to the ITI rating and using a solid<br />
abutment.<br />
5<br />
Buser D et al. Stability of Contour Augmentation and Esthetic Outcomes<br />
of Implant-supported Single Crowns in the Esthetic Z<strong>one</strong>. 3-Year Results of<br />
a Prospective Study With Early Implant Placement Postextraction.<br />
J. Periodontol. 2011;82(3):342–349.<br />
6<br />
Cordaro L et al. Submerged vs non-submerged healing of <strong>implant</strong>s for<br />
single tooth replacement in the aesthetic z<strong>one</strong>: results from a multicentre<br />
RCT. Oral presentation at Annual Meeting of EAO, 2010 in Glasgow (UK).<br />
7<br />
Bornstein M.M. et al. Early loading of non-submerged titanium <strong>implant</strong>s<br />
with a sandblasted and acid-etched surface. 5-year results of a prospective<br />
study in partially edentulous patients. Clin. Oral Implants Res.<br />
2005;16:631–638.<br />
8<br />
Roccuzzo M. et al. Early loading of sandblasted and acid-etched <strong>implant</strong>s:<br />
a randomized-controlled double-blind split-mouth study. Five-year results.<br />
Clin. Oral Implants Res. 2008;19:148–152.<br />
9<br />
Ganeles J, Zöllner A, Jackowski J, ten Bruggenkate C, Beagle J, Guerra<br />
F. Immediate and early loading of Straumann <strong>implant</strong>s with a chemically<br />
modified surface (SLActive ® ) in the posterior mandible and maxilla:<br />
1-year results from a prospective multicenter study. Clin. Oral Impl. Res.<br />
2008;19:1119–1128.<br />
10<br />
Bornstein MM, Wittneben JG, Brägger U, Buser D. Early loading at<br />
21 days of non-submerged titanium <strong>implant</strong>s with a chemically modified<br />
sandblasted and acid-etched surface: 3-year results of a prospective<br />
study in the posterior mandible. J. Periodontol. 2010 Jun;81(6):809–818.<br />
11<br />
Oates TW, Valderrama P, Bischof M, Nedir R, J<strong>one</strong>s A, Simpson J,<br />
Toutenburg H, Cochran DL. Enhanced <strong>implant</strong> stability with a chemically<br />
modified SLA ® surface: a randomized pilot study. The International<br />
Journal of Oral & Maxillofacial Implants. 2007;22(5):755–760.<br />
12<br />
Luongo G, Oteri G. A noninterventional study documenting use and<br />
success of <strong>implant</strong>s with a new chemically modified titanium surface in<br />
daily <strong>dental</strong> practice. J. Oral Implantol. 2010;36(4):305–314.<br />
13<br />
Schwarz F, Herten M, Sager M, Wieland M, Dard M, Becker J. B<strong>one</strong><br />
regeneration in dehiscence-type defects at chemically modified (SLActive)<br />
and conventional SLA titanium <strong>implant</strong>s: A pilot study in dogs. Journal of<br />
Clinical Periodontology. 2007;34(1):78–86.<br />
14<br />
Schwarz F, Ferrari D, Herten M, Mihatovic I, Wieland M, Sager M,<br />
Becker J. Effects of surface hydrophilicity and microtopography on early<br />
stages of soft and hard tissue integration at non-submerged titanium<br />
<strong>implant</strong>s: An immunohistochemical study in dogs. Journal of Periodontology.<br />
2007;78(11):2171–2184.<br />
15<br />
Schwarz F, Herten M, Sager M, Wieland M, Dard M, Becker J.<br />
Histological and immunohistochemical analysis of initial and early<br />
subepithelial connective tissue attachment at chemically modified<br />
and conventional SLA ® titanium <strong>implant</strong>s. A pilot study in dogs. Clinical<br />
Oral Implants Research. 2007;11(3):245–455.<br />
16<br />
Schwarz F, Herten M, Sager M, Wieland M, Dard M, Becker J.<br />
Histological and immunohistochemical analysis of initial and early osseous<br />
integration at chemically modified and conventional SLA ® titanium <strong>implant</strong>s:<br />
Preliminary results of a pilot study in dogs. Clinical Oral Implants Research.<br />
2007;11(4):481–488.<br />
17<br />
Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL,<br />
Hoffmann B, Lussi A, Steinemann SG. Enhanced b<strong>one</strong> apposition to<br />
a chemically modified SLA titanium surface. J. Dent. Res. 2004 Jul;83(7):<br />
529–533.<br />
18<br />
Norm ASTM F67 (states min. tensile strength of annealed titanium).<br />
19<br />
Data on file, used for all Straumann ® titanium and Roxolid ® <strong>implant</strong>s.<br />
3
International Headquarters<br />
Institut Straumann AG<br />
Peter Merian-Weg 12<br />
CH-4002 Basel, Switzerland<br />
Ph<strong>one</strong> +41 (0)61 965 11 11<br />
Fax +41 (0)61 965 11 01<br />
www..straumann.com<br />
Dolder ® is a registered trademark from Prof. Eugen Dolder, former director, School of Dentistry of Zurich. IPS e.max ® is a registered trademark of Ivoclar Vivadent AG,<br />
Liechtenstein. LOCATOR ® is a registered trademark of Zest Anchors, Inc., USA. Some items of the Straumann ® Dental Implant System are not available in all countries.<br />
© Institut Straumann AG, 2012. All rights reserved. Straumann ® and/or other trademarks and logos from Straumann ® menti<strong>one</strong>d herein are the trademarks or registered<br />
trademarks of Straumann Holding AG and/or its affiliates. All rights reserved.<br />
Straumann products are CE marked 03/12 CA152..912/en BG21012