Isostopes
Isostopes
Isostopes
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Isotopes<br />
Name: __________________<br />
Enrichment Activity Skills: interpreting a table, applying<br />
The table below lists isotopes of hydrogen, carbon, and oxygen. Refer to the table to answer the<br />
questions that follow.<br />
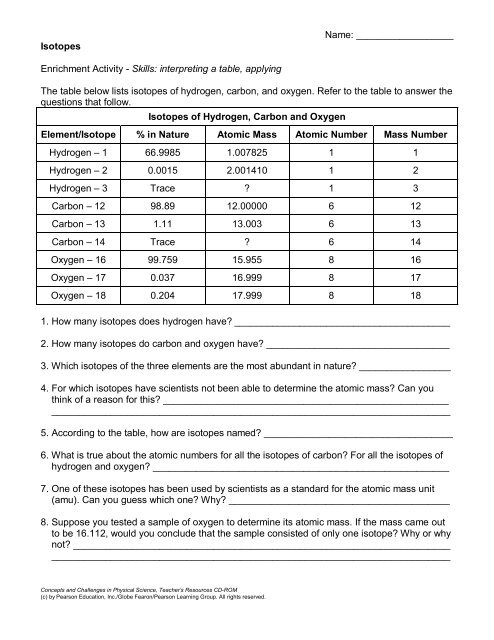
Isotopes of Hydrogen, Carbon and Oxygen<br />
Element/Isotope % in Nature Atomic Mass Atomic Number Mass Number<br />
Hydrogen – 1 66.9985 1.007825 1 1<br />
Hydrogen – 2 0.0015 2.001410 1 2<br />
Hydrogen – 3 Trace ? 1 3<br />
Carbon – 12 98.89 12.00000 6 12<br />
Carbon – 13 1.11 13.003 6 13<br />
Carbon – 14 Trace ? 6 14<br />
Oxygen – 16 99.759 15.955 8 16<br />
Oxygen – 17 0.037 16.999 8 17<br />
Oxygen – 18 0.204 17.999 8 18<br />
1. How many isotopes does hydrogen have? ________________________________________<br />
2. How many isotopes do carbon and oxygen have? __________________________________<br />
3. Which isotopes of the three elements are the most abundant in nature? _________________<br />
4. For which isotopes have scientists not been able to determine the atomic mass? Can you<br />
think of a reason for this? _____________________________________________________<br />
__________________________________________________________________________<br />
5. According to the table, how are isotopes named? ___________________________________<br />
6. What is true about the atomic numbers for all the isotopes of carbon? For all the isotopes of<br />
hydrogen and oxygen? _______________________________________________________<br />
7. One of these isotopes has been used by scientists as a standard for the atomic mass unit<br />
(amu). Can you guess which one? Why? _________________________________________<br />
8. Suppose you tested a sample of oxygen to determine its atomic mass. If the mass came out<br />
to be 16.112, would you conclude that the sample consisted of only one isotope? Why or why<br />
not? ______________________________________________________________________<br />
__________________________________________________________________________<br />
Concepts and Challenges in Physical Science, Teacher’s Resources CDROM<br />
(c) by Pearson Education, Inc./Globe Fearon/Pearson Learning Group. All rights reserved.
Isotopes and Ions<br />
Name: __________________<br />
Directions:<br />
Use the data given to complete the table. Isotopes are also neutral atoms. All atoms of<br />
the same element (e.g. carbon14 and carbon12) are isotopes of that element. Consider<br />
isotopes with mass numbers equal to the mass numbers stated on the periodic table of element<br />
as the neutral atom. Therefore, the isotopes with mass numbers that are different from the<br />
mass numbers stated on the periodic table of elements will be considered isotopes for this<br />
activity. For ions, indicate the positive or negative charge.<br />
Element<br />
Symbol<br />
Atomic<br />
Number<br />
Mass<br />
Number<br />
#<br />
Protons<br />
#<br />
Neutrons<br />
#<br />
Electrons<br />
· Neural Isotope<br />
· + Ion<br />
· Ion<br />
Carbon 6 12 Neutral<br />
Concepts and Challenges in Physical Science, Teacher’s Resources CDROM<br />
(c) by Pearson Education, Inc./Globe Fearon/Pearson Learning Group. All rights reserved.<br />
6 8 6<br />
Oxygen 8 8 10<br />
Mg 12 24 10<br />
Uranium 92 238 Neutral<br />
U 143 92 Isotope<br />
Helium 2 4 2<br />
Na 11 12 10<br />
Chromium 24 52 2+ Ion<br />
Cl 17 18 1 Ion<br />
Hg 80 201 Neutral<br />
Aluminum 13 14 3+ Ion<br />
Ne 20 10 Neutral<br />
1 2 Isotope<br />
1 0 1+ Ion<br />
Phosphorus 15 16 15<br />
Li 4 3 Neutral<br />
Potassium 19 20 19<br />
Fe 26 30 23
Concepts and Challenges in Physical Science, Teacher’s Resources CDROM<br />
(c) by Pearson Education, Inc./Globe Fearon/Pearson Learning Group. All rights reserved.<br />
Name: __________________