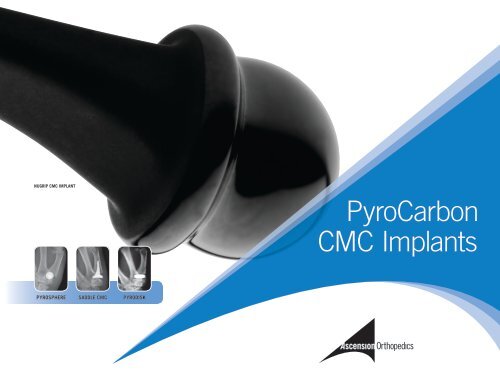
PyroCarbon CMC Implants
PyroCarbon CMC Implants
PyroCarbon CMC Implants
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
nugrip cmc implant<br />
<strong>PyroCarbon</strong><br />
<strong>CMC</strong> <strong>Implants</strong><br />
PyrospherE<br />
saddle cmc<br />
PyroDisk
Raw Graphite<br />
Material<br />
Radiolucent<br />
Carbon Coating<br />
FIGURE 1. Manufacturing Process<br />
CVD Reaction<br />
Chamber<br />
Precision<br />
Machining<br />
Surface<br />
Polishing<br />
Radiopaque<br />
Graphite Substrate<br />
Substrate<br />
Coating<br />
Finished<br />
Implant<br />
FIGURE 2. Elastic modulus of metal and ceramic are<br />
much greater than that of bone or <strong>PyroCarbon</strong>.<br />
<strong>PyroCarbon</strong> Material<br />
<strong>PyroCarbon</strong> is a specific form of carbon that has been tailored for durability and compatibility. <strong>PyroCarbon</strong> has portions<br />
of 2-D and 3-D crystalline structures, resulting in excellent strength and wear properties between those of graphite<br />
and diamond. <strong>PyroCarbon</strong> should not be confused with carbon fibers, which are minute particles used to strengthen<br />
other materials. Manufacturing a <strong>PyroCarbon</strong> implant begins with a precision-machined graphite substrate that<br />
contains 1 atomic percent tungsten to make the core visible on X-ray. Using patented steady-state process controls,<br />
a thick layer of radiolucent On-X ® pure carbon is deposited onto the graphite core, maximizing strength and<br />
durability. FIGURE 1.<br />
The elastic modulus of <strong>PyroCarbon</strong> is very similar to cortical bone resulting in biomechanical compatibility with bone.<br />
Unlike surgical grade metals, <strong>PyroCarbon</strong> transfers load from implant to bone more effectively, thus reducing stress<br />
shielding and potential bone resorption. 1 FIGURE 2.<br />
<strong>PyroCarbon</strong> exhibits exceptional wear performance against bone compared to ceramic and metals. After cyclical testing<br />
to 5,000,000 cycles, <strong>PyroCarbon</strong> demonstrated minimal wear into cortical bone. It was not possible to test the other<br />
materials past 375,000 cycles with Cobalt Chrome, 50,000 cycles with Titanium and 25,000 cycles with Zirconia<br />
because the bone specimens had worn away. 2 FIGURE 3.<br />
Cortical Bone 23<br />
<strong>PyroCarbon</strong><br />
29.4<br />
Titanium<br />
105<br />
Zirconia<br />
210<br />
CoCr Alloy<br />
230<br />
FIGURE 3. Cyclic Wear Test Against Bone<br />
FIGURE 4. Photomicrograph shows<br />
appositional bone growth on material.<br />
FIGURE 5. Left – <strong>PyroCarbon</strong> after 18 months; shows healthy cartilage and surrounding tissue.<br />
Right – metal alloy after 18 months; shows damaged cartilage and tissue.<br />
Wear Penetration Rate into Bone<br />
(nanometer/cycle)<br />
0<br />
10<br />
20<br />
30<br />
40<br />
5,000,000<br />
cycles<br />
0.2<br />
375,000<br />
cycles<br />
4.3<br />
50,000<br />
cycles<br />
30.4<br />
25,000<br />
cycles<br />
34.7<br />
<strong>PyroCarbon</strong> CoCr Titanium Zirconia<br />
Fixation<br />
Pyrocarbon has a micro-porous structure that enhances bone fixation without the need for cement. This unique<br />
fixation is achieved initially through a press fit design using precise instrumentation. Long term fixation is achieved<br />
via appositional bone growth as bone remodels up to the biologically active surface of <strong>PyroCarbon</strong>. 3 FIGURE 4.<br />
Cartilage Friendly<br />
A study of cartilage wear in 45 canine acetabula was performed using both <strong>PyroCarbon</strong> and metal alloy hip prostheses.<br />
<strong>PyroCarbon</strong> showed remarkably less wear damage to cartilage. After 18 months, cartilage articulating with <strong>PyroCarbon</strong><br />
exhibited a 92% survivorship probability compared to 20% survivorship with metal alloys. 4 FIGURE 5.
Ascension <strong>PyroCarbon</strong> <strong>CMC</strong> implants are designed for<br />
high demand patients undergoing joint replacement who:<br />
• Have healthy, strong bones<br />
• Show no signs of joint infection<br />
• Have muscles and tendons which are repairable<br />
Ascension <strong>CMC</strong> <strong>Implants</strong><br />
• Do not have other devices that block implant insertion<br />
or normal motion<br />
• Do not have concerns with cuts healing or other skin<br />
problems<br />
• Do not have numbness or tingling in hands or fingers<br />
The NuGrip <strong>CMC</strong> Implant is our most recent thumb arthroplasty design, bringing together viewpoints and years of expertise<br />
from surgeons around the world. We continue to market multiple <strong>CMC</strong> options, and our challenge is explaining for whom<br />
each implant should be used. Each design allows minimal bone resection and requires no use of cement – providing an open<br />
pathway for revision. All Ascension <strong>PyroCarbon</strong> <strong>CMC</strong> implants require healthy and stable soft tissues for sucessful outcomes.<br />
Stemmed Designs<br />
<strong>CMC</strong> Saddle: STAGES 1 OR 2<br />
Use of this implant requires the full shape of the trapezium to be intact. Minimal lateral erosion of<br />
the trapezium is integral to the functionality of this implant, allowing a secure fit and permitting the<br />
head of the implant to articulate on the trapezium without potentially dislocating radially. No bone<br />
removal on the trapezium is required when using the <strong>CMC</strong> Saddle. The anatomical stem provides a<br />
press fit within the metacarpal for additional stability.<br />
Stages of Arthritis<br />
Based on radiological features, clinical stages of arthritis<br />
were first described by Eaton and Littler, and further defined<br />
in an article by Dr. Sam Anand of the Horton NHS Treatment<br />
Centre and Horton Hospital. These images and descriptions<br />
help identify the proper implant for patient indication.<br />
NUGRIP <strong>CMC</strong>: STAGES 2 OR 3<br />
The NuGrip <strong>CMC</strong> Implant is a redesigned version of the original PyroHemiSphere, which is still<br />
available. New design features include a neck collar and an anatomical stem specifically designed for<br />
the 1st metacarpal to allow press fit and additional stability. The creation of a cup in the trapezium<br />
holds the spherical head in place. This head provides generous range of motion and additional height<br />
– minimizing bone-on-bone impingement.<br />
Interpositional Designs<br />
PYROSPHERE: STAGES 2 OR 3<br />
This device may be used when there is evidence of joint space narrowing and/or sclerosis in the<br />
trapeziometacarpal joint. Shallow cups are made in the metacarpal and trapezium using soft tissues<br />
to stabilize and hold the implant in place. This spherical, interpositional spacer design provides height<br />
to the joint space minimizing potential for impingement and restoring natural range of motion.<br />
PYRODISK: STAGES 2 OR 3 (not available for distribution in the U.S.)<br />
The PyroDisk is a biarticular disk design which incorporates the patient’s own anatomy to restore joint<br />
space and minimize pain. It re-establishes proper alignment of the thumb using the patient’s tendon<br />
for stabilization and preserves trapezial bone stock – only requiring minimal bone resection on the<br />
trapezium and metacarpal – thereby limiting the risk of thumb shortening and weakness.<br />
Stage 1: Synovitis as<br />
evidenced by increased<br />
joint space in X-ray<br />
Stage 3: Sclerosis in the<br />
trapeziometacarpal joint<br />
Stage 2: Joint space<br />
narrowing<br />
Stage 4: Pan trapezial osteoarthritis<br />
as evidenced by radiological<br />
changes in the scapho-trapezialtrapezoidal<br />
(STT) joint.
n joint<br />
n will include<br />
health and<br />
rmed to<br />
in levels of<br />
e on both<br />
edure<br />
thumb joint.<br />
oved. The<br />
n is then closed.<br />
nd send you<br />
rated hand.<br />
ration<br />
ve Motion<br />
rvation for<br />
tients are<br />
ovement.<br />
l be designed<br />
imal stress on<br />
od of recovery.<br />
Notify your<br />
ges in your<br />
throplasty with<br />
rofile that, like<br />
lications are<br />
octor.<br />
Join the thousands of satisfied patients who have<br />
returned to normal and productive lives after<br />
joint replacement surgery using Ascension Orthopedic’s<br />
cutting edge <strong>PyroCarbon</strong> joint implants.<br />
Ask your doctor today how small joint implants<br />
from Ascension Orthopedics can help you.<br />
Ascension thumb implant options:<br />
8700 Cameron Road<br />
Austin, Texas 78754 USA<br />
877-370-5001 (toll-free in U.S.)<br />
512-836-5001<br />
Visit our website at<br />
www.ascensionortho.com/patient<br />
for more information on<br />
<strong>PyroCarbon</strong> joint replacements.<br />
©2010<br />
MK-03-407-001<br />
with <strong>PyroCarbon</strong> implants<br />
Dedicated to transforming<br />
your surgical experience.<br />
This brochure summarizes post-operative<br />
guidelines for Ascension Orthopedics <strong>CMC</strong><br />
Arthroplasty. For more information, contact<br />
customer service at 512-836-5001, or in<br />
the U.S., 1-877-370-5001 (toll-free).<br />
EPL<br />
First Dorsal<br />
interosseous<br />
muscle<br />
APL<br />
Extensor<br />
retinaculum<br />
Flexor<br />
retinaculum<br />
<br />
<br />
<br />
<br />
<br />
Hamate<br />
<br />
<br />
EPB<br />
Proximal Phalanx<br />
Extensor<br />
digitorum tendons<br />
Lumbricals<br />
Abductor digiti<br />
minimi<br />
Flexion<br />
Extension<br />
Distal Phalanx<br />
Metacarpal bones<br />
Flexor digitorum<br />
profundus<br />
Proximal Phalanges<br />
Middle Phalanges<br />
Extensor<br />
carpi ulnaris<br />
Distal Phalanges<br />
Extensor<br />
digiti minimi<br />
Ulna<br />
Extensor digitorum<br />
&<br />
Extensor indicus<br />
Radius<br />
Extensor<br />
retinaculum<br />
Extensor<br />
pollicis longus<br />
Extensor carpi<br />
radialis brevis<br />
Extensor carpi<br />
radialis longus<br />
Extensor<br />
pollicis brevis<br />
Abductor<br />
pollicis longus<br />
SURGEON AUTHOR: Lorenzo Pacelli, MD, Scripps Clinic Torrey Pines, La Jolla, CA<br />
R<br />
R<br />
R<br />
Since this NuGrip case, an enhancement<br />
to the implant design allows for preservation<br />
of approximately two-thirds of the trapezium<br />
during implantation. The new design is<br />
R<br />
now available worldwide.<br />
443-CC01<br />
Patient History<br />
70-year-old female barber complained of severe pain in the right, non-dominant thumb with limited<br />
mobility. There was no inciting event. She rates her pain at 6/10. The pain is aggravated by moderate<br />
activities such as carrying a shopping bag, and she has severe difficulty, or no ability, with many activities<br />
such as playing the piano and using a knife to cut food. Over the course of 24 months, she has failed<br />
to improve despite the treatment of rest and medication. The severe pain and limited range of motion<br />
have left her unable to continue working and performing normal activities. The patient wishes for<br />
definitive treatment to lessen the pain and return to more normal function. Patient had previously<br />
been treated with a NuGrip implant on left <strong>CMC</strong> for osteoarthritis.<br />
Preoperative Assessment / ROM<br />
A physical exam revealed primary diagnosis of osteoarthritis at the base of the thumb. Limited range<br />
of motion noted. Right grip strength averaged at 23 with a lateral pinch of 9. DASH score rated at<br />
52.275. Right opposition measured -1.5 cm to SF DPC. She has no motor or sensory deficits, and<br />
reflexes are normal.<br />
Selected Treatment<br />
This patient is an excellent candidate for <strong>CMC</strong> Arthroplasty. Standard arthroplasty with ceramic<br />
implants shows subsidence of the implants into the metacarpal and trapezium bone. Alternately,<br />
synthetic spacers have been shown to loosen, causing infection and inflammatory response, with even<br />
lower success rates. The Ascension NuGrip <strong>CMC</strong> Implant made of <strong>PyroCarbon</strong> was chosen as<br />
the alternative treatment device. The device is a single component which minimizes bone resections<br />
and preserves the trapezium. The stem is anatomically designed to press fit within the intramedullary<br />
canal without the use of cement. This specifically-designed stem enhances stability and minimizes the<br />
possibility of movement or toggling of the stem within the canal.<br />
Outcome<br />
Arthroplasty was performed using a dorsal approach on the right <strong>CMC</strong> joint. The articular surface of<br />
the metacarpal is excised; the intramedullary canal is prepared with broaches. The canal is prepared<br />
to accept the largest implant that will fit in the metacarpal easily. A centralized cup was made in<br />
the trapezium with a full rim of cortical bone around the cup to properly to seat the head of the<br />
implant allowing full range of motion while limiting the opportunity for subluxation. Interoperative<br />
complications included removal of a bone cyst. Additional bone graft was used to ensure a snug fit<br />
within the intramedullary canal. Patient tolerated the procedure without complications.<br />
3 Months Postoperative<br />
The patient reported improvement in pain intensity 4/10 (compared to 6/10 preoperative). Right<br />
grip strength averaged at 20 with a lateral pinch of 8. DASH score rated at 47.725. Right opposition<br />
measured -2.5cm to SF DPC.<br />
6 Months Postoperative<br />
Patient maintained improvement in pain intensity 4/10. Right grip strength increased with an average<br />
of 20 and maintained a lateral pinch of 8. DASH score rated at 45.25. Normal household activities are<br />
able to be performed with only moderate pain.<br />
9 Months Postoperative<br />
The patient showed continued improvement in pain intensity and denies any significant complaints<br />
while showing excellent range of motion. X-rays reveal adequate longitudinal alignment of both<br />
implants in bilateral thumbs. Right grip strength decreased slightly with an average of 18 while<br />
maintaining a lateral pinch of 8. DASH score rated at 22.725. Normal household activities were<br />
reportedly performed with little or no pain including using a knife to cut food and piano playing.<br />
For More Information<br />
P yro Carbon I m p l a n t s<br />
Durability + Biocompatibility<br />
<strong>PyroCarbon</strong> <strong>Implants</strong> Booklet<br />
The Hand & Wrist<br />
Dorsal View<br />
Volar View<br />
<br />
PIP<br />
Cross Section of Wrist<br />
Ascension NuGrip <strong>CMC</strong> Implant<br />
surgical<br />
technique<br />
Transforming Extremities <br />
CLINICAL CASE<br />
Treatment of Osteoarthritis at the Base of the Thumb<br />
with <strong>PyroCarbon</strong> NuGrip <strong>CMC</strong> Hemiarthroplasty<br />
Additional upper extremity solutions:<br />
First Choice ®<br />
DRUJ System<br />
<strong>PyroCarbon</strong><br />
Lunate<br />
Hold on to<br />
life’s simple<br />
pleasures!<br />
Thumb joint replacement<br />
Patient<br />
Brochure<br />
c m c<br />
Post-Operative<br />
therapy<br />
guidelines<br />
post-operative therapy guidelines Ascension Orthopedics <strong>CMC</strong> Arthroplasty<br />
Therapy<br />
Protocol<br />
Finger Movement<br />
Skeletal View<br />
<br />
<br />
PIP<br />
MCP<br />
<br />
MCP<br />
<br />
<br />
<br />
<br />
First Choice ®<br />
<br />
www.ascensionortho.com <br />
Hand & Wrist Wall Poster<br />
Surgical Techniques<br />
Surgical Videos<br />
Case Reports<br />
Ascension ®<br />
<strong>PyroCarbon</strong><br />
& Silicone<br />
MCP Joints<br />
Ascension ®<br />
<strong>PyroCarbon</strong><br />
& Silicone<br />
PIP Joints<br />
1. Cook SD, Klawitter JJ, Weinstein AM, “The influence of implant elastic modulus on the stress distribution around LTI carbon and aluminum oxide dental implants,” J Biomed Mater Res 1981;15:879-887.<br />
2. Strzepa P, Klawitter JJ, “Ascension <strong>PyroCarbon</strong> Hemisphere Wear Testing Against Bone,” Poster No. 0897, 51st Annual Meeting of the Orthopedic Research Society.<br />
3. Cook SD, Beckenbaugh RD, Weinstein AM, Klawitter JJ, “Pyrolite Carbon <strong>Implants</strong> in the Metacarpophalangeal Joint of Baboons,” Orthopedics, 1983; Vol. 6/No. 8: 952-961.<br />
4. Cook SD, Thomas KA, Kester MA, “Wear Characteristics of the Canine Acetabulum Against Different Femoral Prostheses,” JBJS, 1989; 71-B(2): 189-197.<br />
Ascension Orthopedics, Inc.<br />
8700 Cameron Road<br />
Austin, Texas 78754<br />
512.836.5001 PH<br />
877.370.5001 TF<br />
512.836.6933 FX<br />
customerservice@ascensionortho.com<br />
www.ascensionortho.com<br />
Caution: U.S. federal law restricts these devices<br />
to sale by or on the order of a physician.<br />
MK-03-407-002 rev A<br />
©2011<br />
For more about Ascension <strong>CMC</strong> implants,<br />
visit www.ascensionortho.com/thumb