Half life - Revsworld
Half life - Revsworld
Half life - Revsworld
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
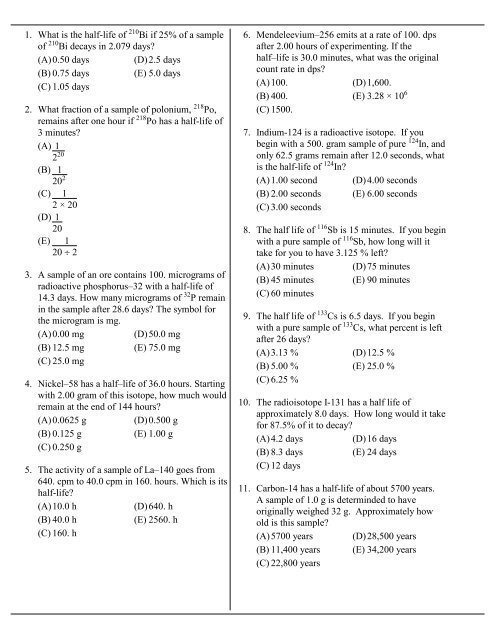
1. What is the half-<strong>life</strong> of 210 Bi if 25% of a sample<br />
of 210 Bi decays in 2.079 days?<br />
(A)0.50 days (D)2.5 days<br />
(B) 0.75 days (E) 5.0 days<br />
(C) 1.05 days<br />
2. What fraction of a sample of polonium, 218 Po,<br />
remains after one hour if 218 Po has a half-<strong>life</strong> of<br />
3 minutes?<br />
(A) 1<br />
2 20<br />
(B) 1<br />
20 2<br />
(C) 1<br />
2 × 20<br />
(D) 1<br />
20<br />
(E) 1<br />
20 ^ 2<br />
3. A sample of an ore contains 100. micrograms of<br />
radioactive phosphorus–32 with a half-<strong>life</strong> of<br />
14.3 days. How many micrograms of 32 P remain<br />
in the sample after 28.6 days? The symbol for<br />
the microgram is mg.<br />
(A)0.00 mg<br />
(D)50.0 mg<br />
(B) 12.5 mg<br />
(E) 75.0 mg<br />
(C) 25.0 mg<br />
4. Nickel–58 has a half–<strong>life</strong> of 36.0 hours. Starting<br />
with 2.00 gram of this isotope, how much would<br />
remain at the end of 144 hours?<br />
(A)0.0625 g (D)0.500 g<br />
(B) 0.125 g<br />
(E) 1.00 g<br />
(C) 0.250 g<br />
5. The activity of a sample of La–140 goes from<br />
640. cpm to 40.0 cpm in 160. hours. Which is its<br />
half-<strong>life</strong>?<br />
(A)10.0 h<br />
(D)640. h<br />
(B) 40.0 h<br />
(E) 2560. h<br />
(C) 160. h<br />
6. Mendeleevium–256 emits at a rate of 100. dps<br />
after 2.00 hours of experimenting. If the<br />
half–<strong>life</strong> is 30.0 minutes, what was the original<br />
count rate in dps?<br />
(A)100.<br />
(D)1,600.<br />
(B) 400. (E) 3.28 × 10 6<br />
(C) 1500.<br />
7. Indium-124 is a radioactive isotope. If you<br />
begin with a 500. gram sample of pure 124 In, and<br />
only 62.5 grams remain after 12.0 seconds, what<br />
is the half-<strong>life</strong> of 124 In?<br />
(A)1.00 second (D)4.00 seconds<br />
(B) 2.00 seconds (E) 6.00 seconds<br />
(C) 3.00 seconds<br />
8. The half <strong>life</strong> of 116 Sb is 15 minutes. If you begin<br />
with a pure sample of 116 Sb, how long will it<br />
take for you to have 3.125 % left?<br />
(A)30 minutes (D)75 minutes<br />
(B) 45 minutes (E) 90 minutes<br />
(C) 60 minutes<br />
9. The half <strong>life</strong> of 133 Cs is 6.5 days. If you begin<br />
with a pure sample of 133 Cs, what percent is left<br />
after 26 days?<br />
(A)3.13 % (D)12.5 %<br />
(B) 5.00 % (E) 25.0 %<br />
(C) 6.25 %<br />
10. The radioisotope I-131 has a half <strong>life</strong> of<br />
approximately 8.0 days. How long would it take<br />
for 87.5% of it to decay?<br />
(A)4.2 days<br />
(D)16 days<br />
(B) 8.3 days<br />
(E) 24 days<br />
(C) 12 days<br />
11. Carbon-14 has a half-<strong>life</strong> of about 5700 years.<br />
A sample of 1.0 g is determinded to have<br />
originally weighed 32 g. Approximately how<br />
old is this sample?<br />
(A)5700 years (D)28,500 years<br />
(B) 11,400 years (E) 34,200 years<br />
(C) 22,800 years
17. What is the missing product in the reaction<br />
12. 131 I has a half-<strong>life</strong> of about 8.1 days. If a lab<br />
(E) 231<br />
93 Np<br />
purchased a sample weighing 10. mg 24.3 days below?<br />
days ago, about how much is left?<br />
234<br />
(A)10. mg<br />
(D)0.6 mg<br />
90 Th → 0 +1β ~ ?<br />
(B) 2.5 mg<br />
(E) 0.3 mg<br />
(A) 234<br />
89 Ac<br />
(C) 1.3 mg<br />
(B) 234<br />
90 Th<br />
(C) 230<br />
90 Ac<br />
13. The half-<strong>life</strong> of 42 K is about 12 hours. It takes<br />
2.0 days for a sample to arrive. How much<br />
(D) 230<br />
89 Ac<br />
(E) 234<br />
91 Pa<br />
should be sent if 5.0 g are needed at the<br />
destination?<br />
18. In the decay series that starts with Fr-222, the<br />
(A)160. g<br />
(D)20. g<br />
first step consists of a beta decay. The next<br />
three steps are alpha particle decay. The final<br />
(B) 80. g<br />
(E) 5.0 g<br />
step is another beta particle decay. What is the<br />
(C) 40. g<br />
product of this decay series?<br />
(A)Pb-212<br />
(D)Bi-210<br />
14. A certain radioactive element undergoes beta<br />
decay to become 214 Bi. What could this be?<br />
(B) Bi-212<br />
(E) Pb-210<br />
(A) 215 Bi<br />
(C) Po-210<br />
(B) 214 Po<br />
(C) 215 19. Base your answer to the following question on<br />
Po<br />
(D) 215 the elements below.<br />
Pb<br />
(E) 214 Pb<br />
(A) Pb<br />
15. Radioactive plutonium, 214<br />
(B) Cd<br />
84<br />
Pu, emits an alpha<br />
(C) U<br />
particle and changes to a new element, “X”. This<br />
(D) Ga<br />
new element emits a beta particle and is<br />
(E) Ni<br />
converted to element “Y”. Which are<br />
representations of elements X and Y?<br />
(A) 210 212<br />
81<br />
X and<br />
83 Y<br />
Which is the end product of the decay of Ra-<br />
226?<br />
(B) 212 210<br />
82 X and 81 Y<br />
(C) 212 211<br />
80<br />
X and<br />
81 Y<br />
(A)A<br />
(D)D<br />
(D) 210 210<br />
80<br />
X and<br />
81 Y<br />
(B) B<br />
(E) E<br />
(E) 210 210<br />
82<br />
X and<br />
83 Y<br />
(C) C<br />
16. Uranium, 235<br />
20. Which of the following choices correctly lists<br />
92<br />
U, emits an alpha particle<br />
alpha particles, beta particles, and gamma rays<br />
producing a daughter nucleus, X. X then<br />
in increasing energy?<br />
disintegrates, emitting a beta particle, and<br />
producing its daughter element, Y. Substance Y<br />
(A)Alpha particles>beta particles>gamma rays<br />
is<br />
(B) Gamma rays>beta particles>alpha particles<br />
(A) 232<br />
89 Ac<br />
(C) Alpha particles>gamma rays>beta particles<br />
(B) 233<br />
91 Pa<br />
(D)Gamma rays>alpha particles>beta particles<br />
(C) 237<br />
93 Np<br />
(E) Beta particles>gamma rays>alpha particles<br />
(D) 231<br />
91 Pa
21. What happens to the mass number and the<br />
atomic number of an element when it undergoes<br />
beta decay?<br />
(A)Neither the mass number nor the atomic<br />
number change.<br />
(B) The mass number does not change and the<br />
atomic number increases by 1.<br />
(C) The mass number does not change and the<br />
atomic number decreases by 2.<br />
(D)The mass number decreases by 4 and the<br />
atomic number decreases by 2.<br />
(E) The mass number increases by 2 and the<br />
atomic number increases by 1.<br />
22. The nuclide 140<br />
56<br />
Ba is radioactive and decays by<br />
loosing 1 beta (î – ) particle. The product formed<br />
is<br />
(A) 140<br />
55 Cs<br />
(B) 140<br />
56 Ba<br />
(C) 141<br />
56 Ba<br />
(D) 140<br />
57 La<br />
(E) 141<br />
57 La<br />
23. What sort of radiation would be expected for 8 5 B?<br />
(A)alpha emission<br />
(B) beta emission<br />
(C) gamma emission<br />
(D)positron emission<br />
(E) none, it is a stable nucleus<br />
24. The radioactive decay 3 H to 3 He occurs by the<br />
process of<br />
(A)beta particle emission<br />
(B) positron emission<br />
(C) alpha particle emission<br />
(D)gamma ray emission<br />
(E) gamma ray absorption<br />
25. Which of the following has the greatest mass?<br />
(A)An alpha particle (D)A positron<br />
(B) A beta particle (E) A hydrogen nucleus<br />
(C) A gamma ray<br />
26. When 12 N decays by positron emission, it<br />
becomes?<br />
(A) 13 N<br />
(B) 12 N<br />
(C) 13 C<br />
(D) 12 C<br />
(E) 8 B<br />
27. If the neutron to proton ratio for an isotope is<br />
too high, the nucleus will achieve stability by<br />
(A)beta decay (D)electron capture<br />
(B) alpha decay (E) gamma decay<br />
(C) positron decay<br />
28. Base your answer to the following question on<br />
the atomic numbers given below.<br />
(A) 9<br />
(B) 10<br />
(C) 46<br />
(D) 51<br />
(E) 84<br />
Which element has no stable isotopes?<br />
(A)A<br />
(D)D<br />
(B) B<br />
(E) E<br />
(C) C<br />
29. Base your answer to the next question on the<br />
following elements.<br />
(A) Germanium<br />
(B) Polonium<br />
(C) Fluorine<br />
(D) Iron<br />
(E) Calcium<br />
Has no stable isotopes.<br />
(A)A<br />
(B) B<br />
(C) C<br />
(D)D<br />
(E) E
Answer Key<br />
1. E<br />
2. A<br />
3. C<br />
4. B<br />
5. B<br />
25. A<br />
26. D<br />
27. A<br />
28. E<br />
29. B<br />
6. D<br />
7. D<br />
8. D<br />
9. C<br />
10. E<br />
11. D<br />
12. C<br />
13. B<br />
14. E<br />
15. E<br />
16. D<br />
17. A<br />
18. D<br />
19. A<br />
20. B<br />
21. B<br />
22. D<br />
23. D<br />
24. A