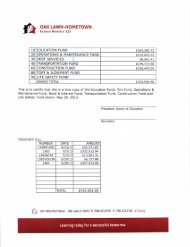
Textbook Unit D (1.1) Atoms are the smallest form of elements.
Textbook Unit D (1.1) Atoms are the smallest form of elements.
Textbook Unit D (1.1) Atoms are the smallest form of elements.
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
KEY CONCEPT<br />
<strong>Atoms</strong> <strong>are</strong> <strong>the</strong> <strong>smallest</strong><br />
<strong>form</strong> <strong>of</strong> <strong>elements</strong>.<br />
BEFORE, you learned<br />
•All matter is made <strong>of</strong> atoms<br />
•Elements <strong>are</strong> <strong>the</strong> simplest<br />
substances<br />
NOW, you will learn<br />
• Where atoms <strong>are</strong> found and<br />
how <strong>the</strong>y <strong>are</strong> named<br />
• About <strong>the</strong> structure <strong>of</strong> atoms<br />
• How ions <strong>are</strong> <strong>form</strong>ed<br />
from atoms<br />
VOCABULARY<br />
proton p. 11<br />
neutron p. 11<br />
nucleus p. 11<br />
electron p. 11<br />
atomic number p. 12<br />
atomic mass number p. 12<br />
isotope p. 12<br />
ion p. 14<br />
EXPLORE The Size <strong>of</strong> <strong>Atoms</strong><br />
How small can you cut paper?<br />
PROCEDURE<br />
1<br />
2<br />
Cut <strong>the</strong> strip <strong>of</strong> paper in half. Cut one <strong>of</strong><br />
<strong>the</strong>se halves in half.<br />
Continue cutting one piece <strong>of</strong> paper in half<br />
as many times as you can.<br />
MATERIALS<br />
• strip <strong>of</strong> paper about<br />
30 centimeters long<br />
•scissors<br />
WHAT DO YOU THINK?<br />
•How many cuts were you able to make?<br />
•Do you think you could keep cutting <strong>the</strong> paper<br />
forever? Why or why not?<br />
reading tip<br />
The word element is<br />
related to elementary,<br />
which means “basic.”<br />
All matter is made <strong>of</strong> atoms.<br />
Think <strong>of</strong> all <strong>the</strong> substances you see and touch every day. Are all <strong>of</strong><br />
<strong>the</strong>se substances <strong>the</strong> same? Obviously, <strong>the</strong> substances that make up<br />
this book you’re reading <strong>are</strong> quite different from <strong>the</strong> substances in<br />
<strong>the</strong> air around you. So how many different substances can <strong>the</strong>re be?<br />
This is a question people have been asking for thousands <strong>of</strong> years.<br />
About 2400 years ago, Greek philosophers proposed that everything<br />
on Earth was made <strong>of</strong> only four basic substances—air, water, fire, and<br />
earth. Everything else contained a mixture <strong>of</strong> <strong>the</strong>se four substances.<br />
As time went on, chemists came to realize that <strong>the</strong>re had to be more<br />
than four basic substances. Today chemists know that about 100 basic<br />
substances, or <strong>elements</strong>, account for everything we see and touch.<br />
Sometimes <strong>the</strong>se <strong>elements</strong> appear by <strong>the</strong>mselves. Most <strong>of</strong>ten, however,<br />
<strong>the</strong>se <strong>elements</strong> appear in combination with o<strong>the</strong>r <strong>elements</strong> to make new<br />
substances. In this section, you’ll learn about <strong>the</strong> atoms <strong>of</strong> <strong>the</strong> <strong>elements</strong><br />
that make up <strong>the</strong> world and how <strong>the</strong>se atoms differ from one ano<strong>the</strong>r.<br />
Chapter 1: Atomic Structure and <strong>the</strong> Periodic Table 9<br />
BD
Atom Concentrations by Mass<br />
Earth’s Crust<br />
Oxygen<br />
46%<br />
Humans<br />
Oxygen<br />
61%<br />
Silicon<br />
28%<br />
O<strong>the</strong>r<br />
12%<br />
Carbon<br />
23%<br />
Iron 5%<br />
Aluminum 8%<br />
Nitrogen 3%<br />
O<strong>the</strong>r 3%<br />
Hydrogen 10%<br />
Types <strong>of</strong> <strong>Atoms</strong> in Earth’s Crust and<br />
Living Things<br />
<strong>Atoms</strong> <strong>of</strong> <strong>the</strong> element hydrogen account for about<br />
90 percent <strong>of</strong> <strong>the</strong> total mass <strong>of</strong> <strong>the</strong> universe.<br />
Hydrogen atoms make up only about 1 percent <strong>of</strong><br />
Earth’s crust, however, and most <strong>of</strong> those hydrogen<br />
atoms <strong>are</strong> combined with oxygen atoms in <strong>the</strong> <strong>form</strong><br />
<strong>of</strong> water.The graph on <strong>the</strong> left shows <strong>the</strong> types <strong>of</strong><br />
atoms in approximately <strong>the</strong> top 100 kilometers <strong>of</strong><br />
Earth’s crust.<br />
The distribution <strong>of</strong> <strong>the</strong> atoms <strong>of</strong> <strong>the</strong> <strong>elements</strong><br />
in living things is very different from what it is in<br />
Earth’s crust. Living things contain at least 25 types<br />
<strong>of</strong> atoms. Although <strong>the</strong> amounts <strong>of</strong> <strong>the</strong>se atoms<br />
vary somewhat, all living things—animals, plants,<br />
and bacteria—<strong>are</strong> composed primarily <strong>of</strong> atoms <strong>of</strong><br />
oxygen, carbon, hydrogen, and nitrogen. As you can<br />
see in <strong>the</strong> lower graph on <strong>the</strong> left, oxygen atoms<br />
account for more than half your body’s mass.<br />
SOURCE: CRC Handbook <strong>of</strong> Chemistry and Physics<br />
Check Your Reading<br />
What is <strong>the</strong> most common element<br />
in <strong>the</strong> universe?<br />
Names and Symbols <strong>of</strong> Elements<br />
Elements get <strong>the</strong>ir names in many different ways. Magnesium, for<br />
example, was named for <strong>the</strong> region in Greece known as Magnesia.<br />
Lithium comes from <strong>the</strong> Greek word lithos, which means “stone.”<br />
Neptunium was named after <strong>the</strong> planet Neptune. The <strong>elements</strong><br />
einsteinium and fermium were named after scientists Albert Einstein<br />
and Enrico Fermi.<br />
Each element has its own unique symbol. For some <strong>elements</strong>,<br />
<strong>the</strong> symbol is simply <strong>the</strong> first letter <strong>of</strong> its name.<br />
hydrogen (H) sulfur (S) carbon (C)<br />
The symbols for o<strong>the</strong>r <strong>elements</strong> use <strong>the</strong> first letter plus one o<strong>the</strong>r<br />
letter <strong>of</strong> <strong>the</strong> element’s name. Notice that <strong>the</strong> first letter is capitalized<br />
but <strong>the</strong> second letter is not.<br />
aluminum (Al) platinum (Pt) cadmium (Cd) zinc (Zn)<br />
The origins <strong>of</strong> some symbols, however, <strong>are</strong> less obvious. The symbol<br />
for gold (Au), for example, doesn’t seem to have anything to do with<br />
<strong>the</strong> element’s name. The symbol refers instead to gold’s name in Latin,<br />
aurum. Lead (Pb), iron (Fe), and copper (Cu) <strong>are</strong> a few o<strong>the</strong>r <strong>elements</strong><br />
whose symbols come from Latin names.<br />
BD 10 <strong>Unit</strong>: Chemical Interactions
Each element is made <strong>of</strong> a different atom.<br />
In <strong>the</strong> early 1800s British scientist John Dalton proposed that each<br />
element is made <strong>of</strong> tiny particles called atoms. Dalton stated that all<br />
<strong>of</strong> <strong>the</strong> atoms <strong>of</strong> a particular element <strong>are</strong> identical but <strong>are</strong> different<br />
from atoms <strong>of</strong> all o<strong>the</strong>r <strong>elements</strong>. Every atom <strong>of</strong> silver, for example, is<br />
similar to every o<strong>the</strong>r atom <strong>of</strong> silver but different from an atom <strong>of</strong> iron.<br />
Dalton’s <strong>the</strong>ory also assumed that atoms could not be divided into<br />
anything simpler. Scientists later discovered that this was not exactly<br />
true. They found that atoms <strong>are</strong> made <strong>of</strong> even smaller particles.<br />
RESOURCE CENTER<br />
CLASSZONE.COM<br />
Learn more about<br />
<strong>the</strong> atom.<br />
The Structure <strong>of</strong> an Atom<br />
A key discovery leading to <strong>the</strong> current model <strong>of</strong> <strong>the</strong> atom was that<br />
atoms contain charged particles. The charge on a particle can be ei<strong>the</strong>r<br />
positive or negative. Particles with <strong>the</strong> same type <strong>of</strong> charge repel each<br />
o<strong>the</strong>r—<strong>the</strong>y <strong>are</strong> pushed apart. Particles with different charges attract<br />
each o<strong>the</strong>r—<strong>the</strong>y <strong>are</strong> drawn toward each o<strong>the</strong>r.<br />
<strong>Atoms</strong> <strong>are</strong> composed <strong>of</strong> three types <strong>of</strong> particles—electrons, protons,<br />
and neutrons. A proton is a positively charged particle, and<br />
a neutron is an uncharged particle. The neutron has approximately<br />
<strong>the</strong> same mass as a proton. The protons and neutrons <strong>of</strong> an atom <strong>are</strong><br />
grouped toge<strong>the</strong>r in <strong>the</strong> atom’s center. This combination <strong>of</strong> protons<br />
and neutrons is called <strong>the</strong> nucleus <strong>of</strong> <strong>the</strong> atom. Because it contains<br />
protons, <strong>the</strong> nucleus has a positive charge. Electrons <strong>are</strong> negatively<br />
charged particles that move around outside <strong>the</strong> nucleus.<br />
VOCABULARY<br />
Remember to make a<br />
frame for neutron, proton,<br />
and electron and for<br />
o<strong>the</strong>r vocabulary terms.<br />
The Atomic Model<br />
<strong>Atoms</strong> <strong>are</strong> made <strong>of</strong> protons, neutrons, and electrons.<br />
FPO<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+<br />
+ +<br />
electron cloud<br />
The electron cloud has<br />
a negative charge.<br />
+<br />
neutron<br />
nucleus<br />
The nucleus has an<br />
overall positive charge.<br />
proton<br />
Particle Charges and Mass<br />
Relative Relative<br />
Particle Mass Charge<br />
Electron 1 –1<br />
Proton 2000 +1<br />
Neutron 2000 0<br />
Which part <strong>of</strong> <strong>the</strong> atom has a negative charge?<br />
Chapter 1: Atomic Structure and <strong>the</strong> Periodic Table 11<br />
BD
SIMULATION<br />
CLASSZONE.COM<br />
Build a model <strong>of</strong><br />
an atom.<br />
<strong>Atoms</strong> <strong>are</strong> extremely small, about 10 –10<br />
meters in diameter. This means that you could<br />
fit millions <strong>of</strong> atoms in <strong>the</strong> period at <strong>the</strong> end<br />
<strong>of</strong> this sentence. The diagram on page 11,<br />
picturing <strong>the</strong> basic structure <strong>of</strong> <strong>the</strong> atom, is<br />
not drawn to scale. In an atom <strong>the</strong> electron<br />
cloud is about 10,000 times <strong>the</strong> diameter <strong>of</strong><br />
<strong>the</strong> nucleus.<br />
Atom Size<br />
Millions <strong>of</strong> atoms<br />
could fit in a space<br />
<strong>the</strong> size <strong>of</strong> this dot.<br />
It would take you<br />
500 years to count<br />
<strong>the</strong> number <strong>of</strong> atoms<br />
in a grain <strong>of</strong> salt.<br />
Electrons <strong>are</strong> much smaller than protons<br />
or neutrons—about 2000 times smaller. Electrons also move about<br />
<strong>the</strong> nucleus very quickly. Scientists have found that it is not possible<br />
to determine <strong>the</strong>ir exact positions with any certainty. This is why we<br />
picture <strong>the</strong> electrons as being in a cloud around <strong>the</strong> nucleus.<br />
The negative electrons remain associated with <strong>the</strong> nucleus because<br />
<strong>the</strong>y <strong>are</strong> attracted to <strong>the</strong> positively charged protons. Also, because<br />
electrical charges that <strong>are</strong> alike (such as two negative charges) repel<br />
each o<strong>the</strong>r, electrons remain spread out in <strong>the</strong> electron cloud. Neutral<br />
atoms have no overall electrical charge because <strong>the</strong>y have an equal<br />
number <strong>of</strong> protons and electrons.<br />
Gold has 79 protons<br />
and 79 electrons.<br />
Atomic Numbers<br />
If all atoms <strong>are</strong> composed <strong>of</strong> <strong>the</strong> same particles, how can <strong>the</strong>re be more<br />
than 100 different <strong>elements</strong>? The identity <strong>of</strong> an atom is determined<br />
by <strong>the</strong> number <strong>of</strong> protons in its nucleus, called <strong>the</strong> atomic number.<br />
Every hydrogen atom—atomic number 1—has exactly one proton in<br />
its nucleus. Every gold atom has 79 protons, which means <strong>the</strong> atomic<br />
number <strong>of</strong> gold is 79.<br />
reading tip<br />
The iso- in isotope is from<br />
<strong>the</strong> Greek language, and it<br />
means “equal.”<br />
Atomic Mass Numbers<br />
The total number <strong>of</strong> protons and neutrons in an atom’s nucleus is<br />
called its atomic mass number. While <strong>the</strong> atoms <strong>of</strong> a certain element<br />
always have <strong>the</strong> same number <strong>of</strong> protons, <strong>the</strong>y may not always have<br />
<strong>the</strong> same number <strong>of</strong> neutrons, so not all atoms <strong>of</strong> an element have <strong>the</strong><br />
same atomic mass number.<br />
All chlorine atoms, for instance, have 17 protons. However, some<br />
chlorine atoms have 18 neutrons, while o<strong>the</strong>r chlorine atoms have<br />
20 neutrons. <strong>Atoms</strong> <strong>of</strong> chlorine with 18 and 20 neutrons <strong>are</strong> called<br />
chlorine isotopes. Isotopes <strong>are</strong> atoms <strong>of</strong> <strong>the</strong> same element that have<br />
a different number <strong>of</strong> neutrons. Some <strong>elements</strong> have many isotopes,<br />
while o<strong>the</strong>r <strong>elements</strong> have just a few.<br />
check your reading<br />
How is atomic mass number different from atomic number?<br />
BD<br />
12 <strong>Unit</strong>: Chemical Interactions
Isotopes<br />
Isotopes have different numbers <strong>of</strong> neutrons.<br />
Chlorine-35<br />
atomic mass number = 35<br />
Chlorine-37<br />
atomic mass number = 37<br />
17 protons<br />
18 neutrons<br />
17 protons<br />
20 neutrons<br />
nucleus<br />
17 electrons<br />
nucleus<br />
17 electrons<br />
A particular isotope is designated by <strong>the</strong> name <strong>of</strong> <strong>the</strong> element and<br />
<strong>the</strong> total number <strong>of</strong> its protons and neutrons. You can find <strong>the</strong> number<br />
<strong>of</strong> neutrons in a particular isotope by subtracting <strong>the</strong> atomic number<br />
from <strong>the</strong> atomic mass number. For example, chlorine-35 indicates <strong>the</strong><br />
isotope <strong>of</strong> chlorine that has 18 neutrons. Chlorine-37 has 20 neutrons.<br />
Every atom <strong>of</strong> a given element always has <strong>the</strong> same atomic number<br />
because it has <strong>the</strong> same number <strong>of</strong> protons. However, <strong>the</strong> atomic mass<br />
number varies depending on <strong>the</strong> number <strong>of</strong> neutrons.<br />
Masses <strong>of</strong> Atomic Particles<br />
How can you model <strong>the</strong> relative masses<br />
<strong>of</strong> atomic particles?<br />
PROCEDURE<br />
1<br />
2<br />
3<br />
Use a paper clip to represent <strong>the</strong> mass <strong>of</strong> an electron. Determine its mass.<br />
Find a substance in <strong>the</strong> classroom (sand, clay, water) from which you could<br />
make a model representing <strong>the</strong> mass <strong>of</strong> a proton or neutron. The mass <strong>of</strong> a<br />
proton or neutron is about 2000 times <strong>the</strong> mass <strong>of</strong> an electron.<br />
Measure out <strong>the</strong> substance until you have enough <strong>of</strong> it to make your model.<br />
SKILL FOCUS<br />
Modeling<br />
MATERIALS<br />
•balance<br />
•large paper clip<br />
• o<strong>the</strong>r items<br />
TIME<br />
20 minutes<br />
WHAT DO YOU THINK?<br />
•What substance did you use to make your model?<br />
•What was <strong>the</strong> model’s mass?<br />
•What do you conclude about <strong>the</strong> masses <strong>of</strong><br />
atomic particles?<br />
CHALLENGE The diameter <strong>of</strong> an electron is approximately<br />
1/2000 that <strong>of</strong> a proton. What two objects could represent<br />
each <strong>of</strong> <strong>the</strong>se to scale?<br />
Chapter 1: Atomic Structure and <strong>the</strong> Periodic Table 13<br />
BD
MAIN IDEA WEB<br />
Make a main idea web to<br />
organize what you know<br />
about ions.<br />
<strong>Atoms</strong> <strong>form</strong> ions.<br />
An atom has an equal number <strong>of</strong> electrons and protons. Since each<br />
electron has one negative charge and each proton has one positive<br />
charge, atoms have no overall electrical charge. An ion is <strong>form</strong>ed when<br />
an atom loses or gains one or more electrons. Because <strong>the</strong> number <strong>of</strong><br />
electrons in an ion is different from <strong>the</strong> number <strong>of</strong> protons, an ion<br />
does have an overall electric charge.<br />
Formation <strong>of</strong> Positive Ions<br />
Consider how a positive ion can be <strong>form</strong>ed from an atom. The left<br />
side <strong>of</strong> <strong>the</strong> illustration below represents a sodium (Na) atom. Its nucleus<br />
contains 11 protons and some neutrons. Because <strong>the</strong> electron cloud<br />
surrounding <strong>the</strong> nucleus consists <strong>of</strong> 11 electrons, <strong>the</strong>re is no overall<br />
charge on <strong>the</strong> atom. If <strong>the</strong> atom loses one electron, however, <strong>the</strong><br />
charges <strong>are</strong> no longer balanced. There is now one more proton than<br />
<strong>the</strong>re <strong>are</strong> electrons. The ion <strong>form</strong>ed, <strong>the</strong>refore, has a positive charge.<br />
11 electrons<br />
(11–)<br />
10 electrons<br />
(10–)<br />
Loses 1<br />
electron<br />
11+ 11+<br />
A positive ion is<br />
smaller than <strong>the</strong><br />
atom that <strong>form</strong>ed<br />
it because it has<br />
fewer electrons.<br />
Sodium Atom (Na)<br />
Sodium Ion (Na + )<br />
Notice <strong>the</strong> size <strong>of</strong> <strong>the</strong> positive ion. Because <strong>the</strong>re <strong>are</strong> fewer electrons,<br />
<strong>the</strong>re is less <strong>of</strong> a repulsion among <strong>the</strong> remaining electrons. Therefore,<br />
<strong>the</strong> positive ion is smaller than <strong>the</strong> neutral atom.<br />
Positive ions <strong>are</strong> represented by <strong>the</strong> symbol for <strong>the</strong> element with a<br />
raised plus sign to indicate <strong>the</strong> positive charge. In <strong>the</strong> above example,<br />
<strong>the</strong> sodium ion is represented as Na + .<br />
Some atoms <strong>form</strong> positive ions by losing more than one electron.<br />
In those cases, <strong>the</strong> symbol for <strong>the</strong> ion also indicates <strong>the</strong> number <strong>of</strong><br />
positive charges on <strong>the</strong> ion. For example, calcium loses two electrons<br />
to <strong>form</strong> an ion Ca 2+ ,and aluminum loses three electrons to <strong>form</strong> Al 3+ .<br />
Check Your Reading<br />
What must happen to <strong>form</strong> a positive ion?<br />
BD 14 <strong>Unit</strong>: Chemical Interactions
Formation <strong>of</strong> Negative Ions<br />
The illustration below shows how a negative ion is <strong>form</strong>ed. In this<br />
case <strong>the</strong> atom is chlorine (Cl). The nucleus <strong>of</strong> a chlorine atom contains<br />
17 protons and some neutrons. The electron cloud has 17 electrons, so<br />
<strong>the</strong> atom has no overall charge. When an electron is added to <strong>the</strong><br />
chlorine atom, a negatively charged ion is <strong>form</strong>ed. Notice that a<br />
negative ion is larger than <strong>the</strong> neutral atom that <strong>form</strong>ed it. The extra<br />
electron increases <strong>the</strong> repulsion within <strong>the</strong> cloud, causing it to expand.<br />
17 electrons<br />
(17–)<br />
18 electrons<br />
(18–)<br />
Gains 1<br />
electron<br />
17+ 17+<br />
A negative ion is<br />
larger than <strong>the</strong><br />
atom that <strong>form</strong>ed<br />
it because it has<br />
more electrons.<br />
Chlorine Atom (Cl) Chloride Ion (Cl – )<br />
Negative ions <strong>are</strong> represented by placing a minus sign to <strong>the</strong> right<br />
and slightly above <strong>the</strong> element’s symbol. The negative chloride ion in<br />
<strong>the</strong> example, <strong>the</strong>refore, would be written as Cl – .Ifan ion has gained<br />
more than one electron, <strong>the</strong> number <strong>of</strong> added electrons is indicated by<br />
a number in front <strong>of</strong> <strong>the</strong> minus sign. Oxygen (O), for example, gains<br />
two electrons when it <strong>form</strong>s an ion. Its symbol is O 2– .<br />
KEY CONCEPTS<br />
1. Which two atoms <strong>are</strong> most<br />
common in Earth’s crust? in<br />
<strong>the</strong> human body?<br />
2. What <strong>are</strong> <strong>the</strong> particles that<br />
make up an atom?<br />
3. What happens when an atom<br />
<strong>form</strong>s an ion?<br />
CRITICAL THINKING<br />
4. Infer Magnesium and sodium<br />
atoms <strong>are</strong> about <strong>the</strong> same size.<br />
How does <strong>the</strong> size <strong>of</strong> a magnesium<br />
ion with a 2+ charge<br />
comp<strong>are</strong> with that <strong>of</strong> a sodium<br />
ion with a single + charge?<br />
5. Comp<strong>are</strong> The atomic number<br />
<strong>of</strong> potassium is 19. How does<br />
potassium-39 differ from<br />
potassium-41?<br />
CHALLENGE<br />
6. Analyze When determining<br />
<strong>the</strong> mass <strong>of</strong> an atom, <strong>the</strong><br />
electrons <strong>are</strong> not considered.<br />
Why can scientists disregard<br />
<strong>the</strong> electrons?<br />
Chapter 1: Atomic Structure and <strong>the</strong> Periodic Table 15<br />
BD