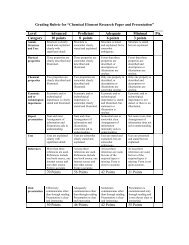
Balancing Chemical Equations Lab
Balancing Chemical Equations Lab
Balancing Chemical Equations Lab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Balancing</strong> <strong>Chemical</strong> <strong>Equations</strong> <strong>Lab</strong><br />
This activity is very useful in helping students to understand the concept of balancing<br />
chemical equations. In 8 th grade, this is a new concept and since I teach all of the students, there<br />
is a range of abilities. Many of the students have difficulty with the concept until we do this lab.<br />
It is a great confidence builder. I purchased the <strong>Balancing</strong> <strong>Equations</strong> Kit (catalog No. AP4577)<br />
from Flinn Scientific and I highly recommend it. Here is a brief description of how it works.<br />
The students work with partners at their tables to build the chemical equations using the colored<br />
discs provided in the kit. I also make small plus and arrow signs for them on cardstock paper.<br />
Pictured below - a sample equation and a picture of what their equation should look like on the<br />
table:<br />
___ Al + ___ O 2 _ ___Al 2 O 3<br />
First, they should build the unbalanced version exactly as it is indicated on the worksheet. It will<br />
look like this:<br />
+<br />
They can easily see that this equation is not balanced since the numbers of blue and pink discs<br />
on the left side of the arrow are not the same as the number on the right side. Remind them that<br />
they have to add a whole unit or molecule to balance the equation. They can never add just one<br />
atom unless that atom is alone in the original unit. They should also be reminded to connect<br />
their atoms so that it is clear how many units or molecules they have when they are done<br />
balancing it.<br />
Their balanced equation should look like this:<br />
+<br />
After building this arrangement of discs on their tables, they can see how many<br />
units are needed to balance the equations and they are prepared to draw a diagram<br />
on their papers and write the equation properly as follows.<br />
4 Al + 3O 2 _ 2Al 2 O 3<br />
Al<br />
Al<br />
Al<br />
Al<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Al<br />
Al<br />
O<br />
O<br />
O<br />
Al<br />
Al<br />
O<br />
O<br />
O<br />
O