Lecture Notes - Mineralogy - Mica Structures
Lecture Notes - Mineralogy - Mica Structures
Lecture Notes - Mineralogy - Mica Structures
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Lecture</strong> <strong>Notes</strong> - <strong>Mineralogy</strong> - <strong>Mica</strong> <strong>Structures</strong><br />
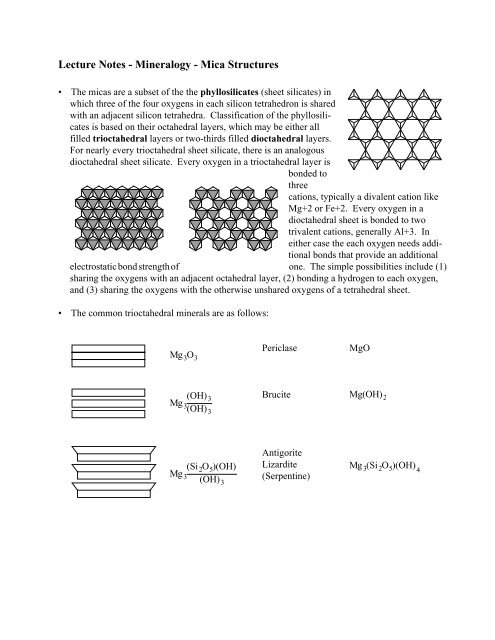
• The micas are a subset of the the phyllosilicates (sheet silicates) in<br />
which three of the four oxygens in each silicon tetrahedron is shared<br />
with an adjacent silicon tetrahedra. Classification of the phyllosilicates<br />
is based on their octahedral layers, which may be either all<br />
filled trioctahedral layers or two-thirds filled dioctahedral layers.<br />
For nearly every trioctahedral sheet silicate, there is an analogous<br />
dioctahedral sheet silicate. Every oxygen in a trioctahedral layer is<br />
bonded to<br />
three<br />
cations, typically a divalent cation like<br />
Mg+2 or Fe+2. Every oxygen in a<br />
dioctahedral sheet is bonded to two<br />
trivalent cations, generally Al+3. In<br />
either case the each oxygen needs additional<br />
bonds that provide an additional<br />
electrostatic bond strength of one. The simple possibilities include (1)<br />
sharing the oxygens with an adjacent octahedral layer, (2) bonding a hydrogen to each oxygen,<br />
and (3) sharing the oxygens with the otherwise unshared oxygens of a tetrahedral sheet.<br />
• The common trioctahedral minerals are as follows:<br />
Mg 3 O 3<br />
Periclase<br />
MgO<br />
(OH) Brucite<br />
Mg 3<br />
Mg(OH) 2<br />
3<br />
(OH) 3<br />
Mg 3<br />
(Si 2 O 5 )(OH)<br />
(OH) 3<br />
Antigorite<br />
Lizardite<br />
(Serpentine)<br />
Mg 3 (Si 2 O 5 )(OH) 4
<strong>Mica</strong> <strong>Structures</strong> 2<br />
Mg 3<br />
(Si 2 O 5 )(OH)<br />
(Si 2 O 5 )(OH)<br />
talc<br />
Mg 3 (Si 4 O 10 )(OH) 2<br />
K+ K+ K+ K+<br />
K+ K+ K+ K+<br />
K+ K+ K+ K+<br />
Phlogopite KMg 3<br />
(AlSi 3<br />
O 10<br />
)(OH) 2<br />
Annite KFe 3<br />
(AlSi 3<br />
O 10<br />
)(OH) 2<br />
Clintonite CaMg 3<br />
(AlSi 3<br />
O 10<br />
)(OH) 2<br />
Siderophyllite K 2<br />
Fe 5<br />
Al(Al 3<br />
Si 5<br />
O 20<br />
)(OH) 4<br />
Eastonite K 2<br />
Mg 5<br />
Al(Al 3<br />
Si 5<br />
O 20<br />
)(OH) 4<br />
K+ K+ K+ K+<br />
• The dioctahedral analogs of these minerals are:<br />
Al 2 O 3<br />
Corundum<br />
[ ]Al 2 O 3<br />
Al 2<br />
(OH) 3<br />
(OH) 3<br />
Gibbsite<br />
[ ]Al 2 (OH) 6<br />
Al 2<br />
(Si 2 O 5 )(OH)<br />
(OH) 3<br />
Kaolinite<br />
[ ]Al 2 (Si 2 O 5 )(OH) 4<br />
Al 2<br />
(Si 2 O 5 )(OH)<br />
(Si 2 O 5 )(OH)<br />
Pyrophyllite<br />
[ ]Al 2 (Si 4 O 10 )(OH) 2
<strong>Mica</strong> <strong>Structures</strong> 3<br />
K+ K+ K+ K+<br />
K+ K+ K+ K+<br />
Muscovite K[ ]Al 2<br />
(AlSi 3<br />
O 10<br />
)(OH) 2<br />
Paragonite Na[ ]Al 2<br />
(AlSi 3<br />
O 10<br />
)(OH) 2<br />
Margarite Ca [ ]Al 2<br />
(AlSi 3<br />
O 10<br />
)(OH) 2<br />
Lepidolite K 2<br />
[ ](Li,Al) 5<br />
(Al 2<br />
Si 6<br />
O 20<br />
)(OH,F) 4<br />
K+ K+ K+ K+<br />
K+ K+ K+ K+<br />
Structure of phlogopite viewed approximately perpendicular to c as drawn by CrystalMaker
<strong>Mica</strong> <strong>Structures</strong> 4<br />
Structure of phlogopite viewed perpendicular to (001) as drawn by CrystalMaker ©.