Solubility behavior of amphiphilic block and random copolymers ...
Solubility behavior of amphiphilic block and random copolymers ...
Solubility behavior of amphiphilic block and random copolymers ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
AMPHIPHILIC BLOCK AND RANDOM COPOLYMERS 521<br />
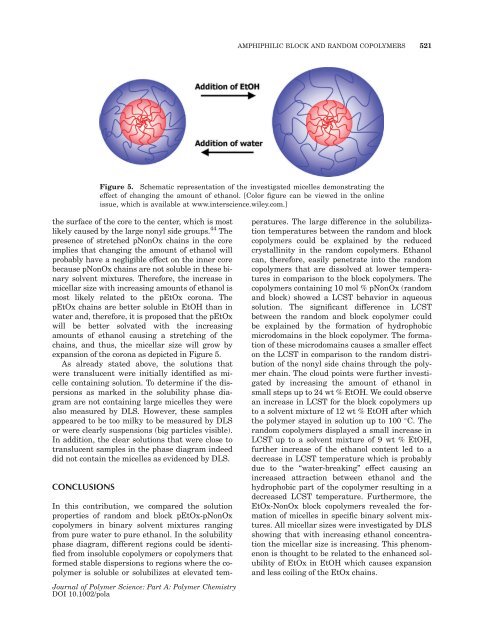
Figure 5. Schematic representation <strong>of</strong> the investigated micelles demonstrating the<br />
effect <strong>of</strong> changing the amount <strong>of</strong> ethanol. [Color figure can be viewed in the online<br />
issue, which is available at www.interscience.wiley.com.]<br />
the surface <strong>of</strong> the core to the center, which is most<br />
likely caused by the large nonyl side groups. 44 The<br />
presence <strong>of</strong> stretched pNonOx chains in the core<br />
implies that changing the amount <strong>of</strong> ethanol will<br />
probably have a negligible effect on the inner core<br />
because pNonOx chains are not soluble in these binary<br />
solvent mixtures. Therefore, the increase in<br />
micellar size with increasing amounts <strong>of</strong> ethanol is<br />
most likely related to the pEtOx corona. The<br />
pEtOx chains are better soluble in EtOH than in<br />
water <strong>and</strong>, therefore, it is proposed that the pEtOx<br />
will be better solvated with the increasing<br />
amounts <strong>of</strong> ethanol causing a stretching <strong>of</strong> the<br />
chains, <strong>and</strong> thus, the micellar size will grow by<br />
expansion <strong>of</strong> the corona as depicted in Figure 5.<br />
As already stated above, the solutions that<br />
were translucent were initially identified as micelle<br />
containing solution. To determine if the dispersions<br />
as marked in the solubility phase diagram<br />
are not containing large micelles they were<br />
also measured by DLS. However, these samples<br />
appeared to be too milky to be measured by DLS<br />
or were clearly suspensions (big particles visible).<br />
In addition, the clear solutions that were close to<br />
translucent samples in the phase diagram indeed<br />
did not contain the micelles as evidenced by DLS.<br />
CONCLUSIONS<br />
Journal <strong>of</strong> Polymer Science: Part A: Polymer Chemistry<br />
DOI 10.1002/pola<br />
In this contribution, we compared the solution<br />
properties <strong>of</strong> r<strong>and</strong>om <strong>and</strong> <strong>block</strong> pEtOx-pNonOx<br />
<strong>copolymers</strong> in binary solvent mixtures ranging<br />
from pure water to pure ethanol. In the solubility<br />
phase diagram, different regions could be identified<br />
from insoluble <strong>copolymers</strong> or <strong>copolymers</strong> that<br />
formed stable dispersions to regions where the copolymer<br />
is soluble or solubilizes at elevated temperatures.<br />
The large difference in the solubilization<br />
temperatures between the r<strong>and</strong>om <strong>and</strong> <strong>block</strong><br />
<strong>copolymers</strong> could be explained by the reduced<br />
crystallinity in the r<strong>and</strong>om <strong>copolymers</strong>. Ethanol<br />
can, therefore, easily penetrate into the r<strong>and</strong>om<br />
<strong>copolymers</strong> that are dissolved at lower temperatures<br />
in comparison to the <strong>block</strong> <strong>copolymers</strong>. The<br />
<strong>copolymers</strong> containing 10 mol % pNonOx (r<strong>and</strong>om<br />
<strong>and</strong> <strong>block</strong>) showed a LCST <strong>behavior</strong> in aqueous<br />
solution. The significant difference in LCST<br />
between the r<strong>and</strong>om <strong>and</strong> <strong>block</strong> copolymer could<br />
be explained by the formation <strong>of</strong> hydrophobic<br />
microdomains in the <strong>block</strong> copolymer. The formation<br />
<strong>of</strong> these microdomains causes a smaller effect<br />
on the LCST in comparison to the r<strong>and</strong>om distribution<br />
<strong>of</strong> the nonyl side chains through the polymer<br />
chain. The cloud points were further investigated<br />
by increasing the amount <strong>of</strong> ethanol in<br />
small steps up to 24 wt % EtOH. We could observe<br />
an increase in LCST for the <strong>block</strong> <strong>copolymers</strong> up<br />
to a solvent mixture <strong>of</strong> 12 wt % EtOH after which<br />
the polymer stayed in solution up to 100 C. The<br />
r<strong>and</strong>om <strong>copolymers</strong> displayed a small increase in<br />
LCST up to a solvent mixture <strong>of</strong> 9 wt % EtOH,<br />
further increase <strong>of</strong> the ethanol content led to a<br />
decrease in LCST temperature which is probably<br />
due to the ‘‘water-breaking’’ effect causing an<br />
increased attraction between ethanol <strong>and</strong> the<br />
hydrophobic part <strong>of</strong> the copolymer resulting in a<br />
decreased LCST temperature. Furthermore, the<br />
EtOx-NonOx <strong>block</strong> <strong>copolymers</strong> revealed the formation<br />
<strong>of</strong> micelles in specific binary solvent mixtures.<br />
All micellar sizes were investigated by DLS<br />
showing that with increasing ethanol concentration<br />
the micellar size is increasing. This phenomenon<br />
is thought to be related to the enhanced solubility<br />
<strong>of</strong> EtOx in EtOH which causes expansion<br />
<strong>and</strong> less coiling <strong>of</strong> the EtOx chains.