Actemra (tocilizumab) Product Information (PI) - Roche Australia
Actemra (tocilizumab) Product Information (PI) - Roche Australia
Actemra (tocilizumab) Product Information (PI) - Roche Australia
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
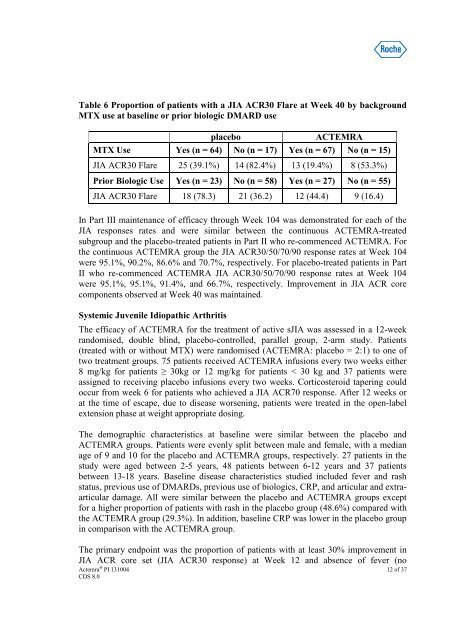
Table 6 Proportion of patients with a JIA ACR30 Flare at Week 40 by background<br />
MTX use at baseline or prior biologic DMARD use<br />
placebo<br />
ACTEMRA<br />
MTX Use Yes (n = 64) No (n = 17) Yes (n = 67) No (n = 15)<br />
JIA ACR30 Flare 25 (39.1%) 14 (82.4%) 13 (19.4%) 8 (53.3%)<br />
Prior Biologic Use Yes (n = 23) No (n = 58) Yes (n = 27) No (n = 55)<br />
JIA ACR30 Flare 18 (78.3) 21 (36.2) 12 (44.4) 9 (16.4)<br />
In Part III maintenance of efficacy through Week 104 was demonstrated for each of the<br />
JIA responses rates and were similar between the continuous ACTEMRA-treated<br />
subgroup and the placebo-treated patients in Part II who re-commenced ACTEMRA. For<br />
the continuous ACTEMRA group the JIA ACR30/50/70/90 response rates at Week 104<br />
were 95.1%, 90.2%, 86.6% and 70.7%, respectively. For placebo-treated patients in Part<br />
II who re-commenced ACTEMRA JIA ACR30/50/70/90 response rates at Week 104<br />
were 95.1%, 95.1%, 91.4%, and 66.7%, respectively. Improvement in JIA ACR core<br />
components observed at Week 40 was maintained.<br />
Systemic Juvenile Idiopathic Arthritis<br />
The efficacy of ACTEMRA for the treatment of active sJIA was assessed in a 12-week<br />
randomised, double blind, placebo-controlled, parallel group, 2-arm study. Patients<br />
(treated with or without MTX) were randomised (ACTEMRA: placebo = 2:1) to one of<br />
two treatment groups. 75 patients received ACTEMRA infusions every two weeks either<br />
8 mg/kg for patients ≥ 30kg or 12 mg/kg for patients < 30 kg and 37 patients were<br />
assigned to receiving placebo infusions every two weeks. Corticosteroid tapering could<br />
occur from week 6 for patients who achieved a JIA ACR70 response. After 12 weeks or<br />
at the time of escape, due to disease worsening, patients were treated in the open-label<br />
extension phase at weight appropriate dosing.<br />
The demographic characteristics at baseline were similar between the placebo and<br />
ACTEMRA groups. Patients were evenly split between male and female, with a median<br />
age of 9 and 10 for the placebo and ACTEMRA groups, respectively. 27 patients in the<br />
study were aged between 2-5 years, 48 patients between 6-12 years and 37 patients<br />
between 13-18 years. Baseline disease characteristics studied included fever and rash<br />
status, previous use of DMARDs, previous use of biologics, CRP, and articular and extraarticular<br />
damage. All were similar between the placebo and ACTEMRA groups except<br />
for a higher proportion of patients with rash in the placebo group (48.6%) compared with<br />
the ACTEMRA group (29.3%). In addition, baseline CRP was lower in the placebo group<br />
in comparison with the ACTEMRA group.<br />
The primary endpoint was the proportion of patients with at least 30% improvement in<br />
JIA ACR core set (JIA ACR30 response) at Week 12 and absence of fever (no<br />
<strong>Actemra</strong> ® <strong>PI</strong> 131004 12 of 37<br />
CDS 8.0