phys4.15 Page 1
phys4.15 Page 1
phys4.15 Page 1
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Stimulated Emission of Radiation<br />
- stimulated emission is referring to the emission of radiation (a photon) from one quantum<br />
system at its transition frequency induced by the presence of other photons at that frequency<br />
- introduced by Einstein in 1917 to derive Planck's blackbody radiation law<br />
- is the basis for the operation of a LASER (Light Amplification by Stimulated Emission of<br />
Radiation)<br />
Consider an assembly of N atoms with energy levels E i and E j in<br />
thermal equilibrium with a thermal radiation field with energy<br />
density u(ν) at temperature T<br />
- the transition frequency ν ij in the individual atom is<br />
- the probability of an atom in state i to absorb a photon is<br />
proportional to u(ν ij ) (related to the number of photons at that<br />
frequency) and to the probability B ij (Einstein B coefficient) of<br />
the atom to absorb a photon at that frequency<br />
<strong>phys4.15</strong> <strong>Page</strong> 1<br />
- thus, the total number of atoms per second that absorb a photon is<br />
where N i is the number of atoms in state i<br />
- an atom in state j has a probability A ji (Einstein A coefficient) to<br />
spontaneously decay into state i by emitting a photon at frequency ν in a<br />
process called spontaneous emission<br />
- also, an atom in state j has a probability B ji to decay into state i by<br />
emitting a photon at frequency ν ij stimulated by the presence of other<br />
photons at that frequency the number of which is proportional to u(ν ij )<br />
- this process is called stimulated emission<br />
- thus the total number N ji of atoms per second that make a transition into<br />
the lower state is given by<br />
- in thermal equilibrium the number of atoms that make a transition to lower energy<br />
must be identical to the number of atoms making a transition to higher energy<br />
<strong>phys4.15</strong> <strong>Page</strong> 2
Transitions in a collection of atoms<br />
interacting with a radiation field<br />
- in thermal equilibrium<br />
- solve for the energy density of the radiation field u(ν)<br />
- with the number of atoms in the ground N i or excited state N j is given by classical M.-B.<br />
statistics<br />
- thus<br />
<strong>phys4.15</strong> <strong>Page</strong> 3<br />
- hence the energy density of a radiation field in thermal equilibrium is given by<br />
- this is Planck's formula for blackbody radiation for<br />
- for identical upwards and downwards transition rates<br />
- and with the ratio of spontaneous emission to<br />
induced emission rate given by<br />
note:<br />
- stimulated emission does occur and is consistent with the form of the<br />
blackbody spectrum<br />
- the probability for stimulated absorption is equal to the probability<br />
stimulated emission<br />
- the likelihood of spontaneous emission relative to stimulated emission<br />
increases rapidly with frequency<br />
- knowing one of the parameters A ji , B ji , B ij we can determine the other<br />
ones<br />
- spontaneous emission is 'stimulated' by vacuum fluctuations (QED)<br />
<strong>phys4.15</strong> <strong>Page</strong> 4
Light Amplification by Stimulated Emission of Radiation (LASER)<br />
LASER<br />
Light Amplification by Stimulated Emission of Radiation<br />
properties:<br />
• monochromatic<br />
• coherent<br />
• directed<br />
• bright<br />
<strong>phys4.15</strong> <strong>Page</strong> 5<br />
Properties of LASER light<br />
monochromatic<br />
- relative line width<br />
coherence<br />
- temporal coherence<br />
- spatial coherence limited only by optics<br />
brightness<br />
- intensities<br />
- large photon flux<br />
in pulsed operation<br />
- short pulses<br />
<strong>phys4.15</strong> <strong>Page</strong> 6
The LASER<br />
1. active LASER medium (gas, dye, solid)<br />
2. pumping (light, electrical energy)<br />
3. 100% reflecting mirror<br />
4. coupling mirror (< 1% transmission)<br />
5. LASER beam<br />
<strong>phys4.15</strong> <strong>Page</strong> 7<br />
The LASER Principle<br />
- generation of a large number of excitations in a medium<br />
by (stimulated) absorption<br />
- a long lived metastable state to achieve population inversion<br />
- stimulated emission of radiation<br />
- storage of radiation field in a resonant cavity with<br />
partially transmitting mirror<br />
<strong>phys4.15</strong> <strong>Page</strong> 8
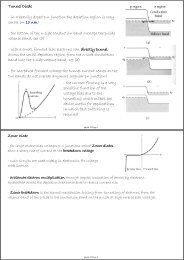
Generation of Population Inversion<br />
- excitation by optical pumping in a three level system<br />
- transition (possibly non-radiative) to a metastable state<br />
- stimulated emission<br />
- at least a three level system is required to achieve<br />
population inversion as the symmetry between the<br />
probability for absorption (B ij ) and emission (B ji ) results<br />
at best in equal population of the two states such that<br />
emission cannot be the dominant process (i.e. there would<br />
be no amplification in number of photons)<br />
<strong>phys4.15</strong> <strong>Page</strong> 9<br />
Examples of Lasers<br />
Ruby Laser: Cr + ions in Al 2 O 3 crystal (sapphire)<br />
- optical pumping using flash light<br />
- Helium-Neon Laser: gas mixture (90 % He, 10 % Ne) where He is excited electrically in gas<br />
discharge and energy is transferred in collision to Ne<br />
<strong>phys4.15</strong> <strong>Page</strong> 10
Nobel Prize in Physics 1964<br />
for fundamental work in the field of quantum electronics,<br />
which has led to the construction of oscillators and<br />
amplifiers based on the maser-laser principle<br />
Charles H. Townes Nicolay G. Basov Aleksandr M. Prokhorov<br />
<strong>phys4.15</strong> <strong>Page</strong> 11<br />
Laser-Pointer<br />
• housing<br />
• electronics<br />
• LASER<br />
and optics<br />
<strong>phys4.15</strong> <strong>Page</strong> 12
CD Player<br />
• compact disc<br />
• the mechanism<br />
• portable CD player<br />
(older modell)<br />
<strong>phys4.15</strong> <strong>Page</strong> 13<br />
Laser-Printer<br />
• resolution up to 20 µm or 50 points per mm (= 1270 dpi)<br />
• colors with multiple stages<br />
<strong>phys4.15</strong> <strong>Page</strong> 14
Material Processing<br />
• CO 2 gas LASER<br />
• up to 20 kW power<br />
www.trumpf.com<br />
<strong>phys4.15</strong> <strong>Page</strong> 15<br />
Lasers in Medicine<br />
• eye surgery<br />
• dermatology etc.<br />
<strong>phys4.15</strong> <strong>Page</strong> 16
Specific Heat of Solids<br />
- dependence of thermal energy stored in a solid on temperature<br />
- molar specific heat at constant volume c V : energy that needs to be added to 1 mol of a solid<br />
to increase its temperature by 1 Kelvin<br />
- thermal energy is stored in solids in the vibrations of its constituents (atoms, ions or<br />
molecules)<br />
- in a simple model the atoms can vibrate along 3 independent (x, y, z) directions that each<br />
can be considered as an independent harmonic oscillator<br />
- the equipartition theorem tells us the average energy stored in each degree of freedom, thus<br />
the total energy of a solid containing 1 mol = N 0 = 6.022 10 23 of atoms is given by<br />
- R = 8.31 J/mol is the ideal gas constant (pV = nRT)<br />
- specific heat (Dulong Petit law)<br />
<strong>phys4.15</strong> <strong>Page</strong> 17<br />
Dulong petit law fails for light elements (e.g.<br />
carbon) and at low temperatures, see plot<br />
- problem can be solved using the Bose-Einstein<br />
distribution function for the probability of an<br />
harmonic oscillator to have energy hν<br />
- average energy for a harmonic oscillator of frequency ν<br />
- total energy per mol<br />
- thus Einstein's specific heat formula is<br />
<strong>phys4.15</strong> <strong>Page</strong> 18
- the high temperature limit of Einstein's formula<br />
- therefore<br />
- at high temperatures the thermal energy is much larger than the typical level separation of<br />
the harmonic oscillators in the solid and the specific heat is close to the classical prediction<br />
- at low temperatures the thermal energy is comparable to the level separation and quantum<br />
statistics start to play a role<br />
- for lighter elements with smaller masses the oscillator frequencies are higher and thus<br />
quantum effects are more important even at higher temperatures<br />
- the zero point motion does not play a role for the specific heat as it is only a constant offset<br />
in energy<br />
<strong>phys4.15</strong> <strong>Page</strong> 19<br />
Debeye theory<br />
- Debeye regarded a solid as a continuous elastic medium with elastic standing waves<br />
instead of considering the atoms as individual oscillators the way Einstein did<br />
- these standing waves can have frequencies ν up to a maximum value ν max<br />
- they are like transverse and longitudinal elastic waves that propagate at the speed of<br />
sound in the solid<br />
- their energies are quantized as in a harmonic oscillator<br />
- each quantum of the elastic wave is called a phonon<br />
- Debeye assumed that 3N different modes exist per mol in a solid<br />
- the resulting formula describes the specific heat of the solid better than Einstein's<br />
formula<br />
- to be discussed in detail in solid state physiscs<br />
<strong>phys4.15</strong> <strong>Page</strong> 20