Dissociative electron attachment to the unstable carbon ...
Dissociative electron attachment to the unstable carbon ...
Dissociative electron attachment to the unstable carbon ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Dissociative</strong> <strong>electron</strong> <strong>attachment</strong> <strong>to</strong> CS 7<br />
Counts<br />
18000<br />
16000<br />
14000<br />
12000<br />
10000<br />
8000<br />
6000<br />
4000<br />
2000<br />
0<br />
(a)<br />
CS +<br />
CS 2<br />
+<br />
10 12 14 16 18 20<br />
Electron Energy (eV)<br />
Counts<br />
25000<br />
20000<br />
15000<br />
10000<br />
5000<br />
0<br />
(b)<br />
CS +<br />
CS 2<br />
+<br />
10 12 14 16 18 20<br />
Electron Energy (eV)<br />
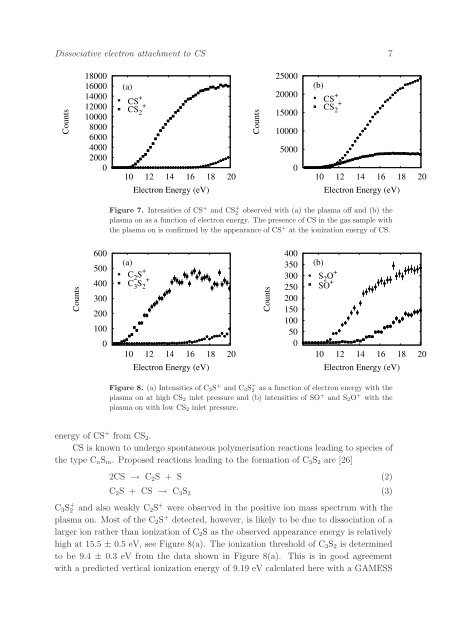
Figure 7. Intensities of CS + and CS + 2 observed with (a) <strong>the</strong> plasma off and (b) <strong>the</strong><br />
plasma on as a function of <strong>electron</strong> energy. The presence of CS in <strong>the</strong> gas sample with<br />
<strong>the</strong> plasma on is confirmed by <strong>the</strong> appearance of CS + at <strong>the</strong> ionization energy of CS.<br />
Counts<br />
600<br />
500<br />
400<br />
300<br />
200<br />
100<br />
0<br />
(a)<br />
C 2 S +<br />
C 3 S 2<br />
+<br />
10 12 14 16 18 20<br />
Electron Energy (eV)<br />
Counts<br />
400<br />
350<br />
300<br />
250<br />
200<br />
150<br />
100<br />
50<br />
0<br />
(b)<br />
S 2 O +<br />
SO +<br />
10 12 14 16 18 20<br />
Electron Energy (eV)<br />
Figure 8. (a) Intensities of C 2 S + and C 3 S + 2 as a function of <strong>electron</strong> energy with <strong>the</strong><br />
plasma on at high CS 2 inlet pressure and (b) intensities of SO + and S 2 O + with <strong>the</strong><br />
plasma on with low CS 2 inlet pressure.<br />
energy of CS + from CS 2 .<br />
CS is known <strong>to</strong> undergo spontaneous polymerisation reactions leading <strong>to</strong> species of<br />
<strong>the</strong> type C n S m . Proposed reactions leading <strong>to</strong> <strong>the</strong> formation of C 3 S 2 are [26]<br />
2CS → C 2 S + S (2)<br />
C 2 S + CS → C 3 S 2 (3)<br />
C 3 S + 2 and also weakly C 2 S + were observed in <strong>the</strong> positive ion mass spectrum with <strong>the</strong><br />
plasma on. Most of <strong>the</strong> C 2 S + detected, however, is likely <strong>to</strong> be due <strong>to</strong> dissociation of a<br />
larger ion ra<strong>the</strong>r than ionization of C 2 S as <strong>the</strong> observed appearance energy is relatively<br />
high at 15.5 ± 0.5 eV, see Figure 8(a). The ionization threshold of C 3 S 2 is determined<br />
<strong>to</strong> be 9.4 ± 0.3 eV from <strong>the</strong> data shown in Figure 8(a). This is in good agreement<br />
with a predicted vertical ionization energy of 9.19 eV calculated here with a GAMESS