identification and analysis of drugs in the solid state by 13c cpmas nmr
identification and analysis of drugs in the solid state by 13c cpmas nmr
identification and analysis of drugs in the solid state by 13c cpmas nmr
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Identification <strong>and</strong> <strong>analysis</strong> <strong>of</strong> <strong>drugs</strong> <strong>in</strong> <strong>the</strong> <strong>solid</strong> <strong>state</strong> <strong>by</strong>... 299<br />
Table 1. 13 C chemical shifts (δ, ppm) for carbons <strong>of</strong> suxamethonium chloride <strong>in</strong> solution (DMSO-d 6 ) <strong>and</strong> <strong>solid</strong> <strong>state</strong>, <strong>and</strong> cross polarization<br />
parameters (ms) as obta<strong>in</strong>ed us<strong>in</strong>g two CP models.<br />
C Carbon type Model I-S Model I*I-S<br />
δ solution δ CPMAS T CP T 1ρ T 2 T df<br />
1,8 63,47 63,5 CH 2 ñ 4.1 ± 0.2 2.6 ± 0.1 22.7 ± 1.6<br />
2,7 58,06 59,3 CH 2 ñ 3.9 ± 0.1 2.4 ± 0.2 19.4 ± 1.2<br />
3,6 171,43 173,5 C=O 1.09 ± 0.07 4.6 ± 0.2 ñ ñ<br />
4,5 28,54 29,4 CH 2<br />
CH 3 52,79 53,8 NCH 3 0.22 ± 0.01 4.1 ± 0.2 ñ ñ<br />
Table 2. 13 C NMR chemical shifts (δ, ppm) <strong>of</strong> cortisone hemisucc<strong>in</strong>ate<br />
<strong>in</strong> solution (acetone-d 6 ) <strong>and</strong> <strong>solid</strong> <strong>state</strong>; <strong>the</strong> differences<br />
∆= δ solution ñ δ CPMAS >1 ppm.<br />
C δ solution δ CPMAS ∆<br />
1 32.26 32.8<br />
2 33.46 32.8<br />
3 204.69 201.7 3.0<br />
4 121.39 121.9<br />
5 175.34 175.0<br />
6 32.43 32.8<br />
7 31.48 32.8 -1.3<br />
8 30.52 32.8 -2.3<br />
9 56.09 56.4<br />
10 39.05 39.5<br />
11 69.06 68.0<br />
12 39.57 39.5<br />
13 47.62 47.4<br />
14 52.48 52.3<br />
15 23.56 23.5<br />
16 32.99 32.8<br />
17 90.13 89.5<br />
18 16.78 17.2<br />
19 20.78 20.6<br />
20 209.28 209.0<br />
21 68.12 68.0<br />
succ<strong>in</strong>ate anion<br />
a 176,08 179.6 -3.5<br />
b 28,96 30<br />
c 28,67 30<br />
d 170,94 172.2 -1.2<br />
a<br />
b<br />
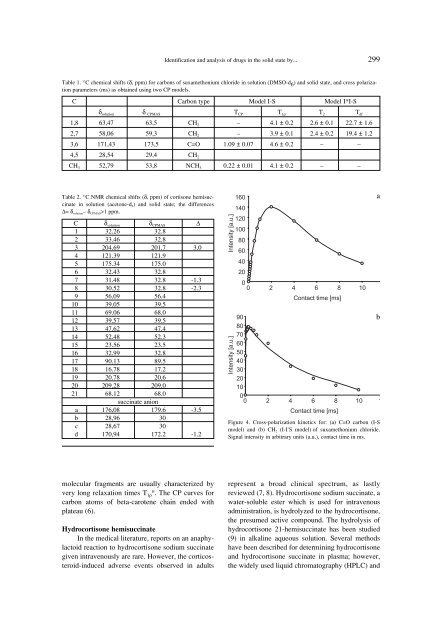
Figure 4. Cross-polarization k<strong>in</strong>etics for: (a) C=O carbon (I-S<br />
model) <strong>and</strong> (b) CH 2 (I-I * S model) <strong>of</strong> suxamethonium chloride.<br />
Signal <strong>in</strong>tensity <strong>in</strong> arbitrary units (a.u.), contact time <strong>in</strong> ms.<br />
molecular fragments are usually characterized <strong>by</strong><br />
very long relaxation times T 1ρH . The CP curves for<br />
carbon atoms <strong>of</strong> beta-carotene cha<strong>in</strong> ended with<br />
plateau (6).<br />
Hydrocortisone hemisucc<strong>in</strong>ate<br />
In <strong>the</strong> medical literature, reports on an anaphylactoid<br />
reaction to hydrocortisone sodium succ<strong>in</strong>ate<br />
given <strong>in</strong>travenously are rare. However, <strong>the</strong> corticosteroid-<strong>in</strong>duced<br />
adverse events observed <strong>in</strong> adults<br />
represent a broad cl<strong>in</strong>ical spectrum, as lastly<br />
reviewed (7, 8). Hydrocortisone sodium succ<strong>in</strong>ate, a<br />
water-soluble ester which is used for <strong>in</strong>travenous<br />
adm<strong>in</strong>istration, is hydrolyzed to <strong>the</strong> hydrocortisone,<br />
<strong>the</strong> presumed active compound. The hydrolysis <strong>of</strong><br />
hydrocortisone 21-hemisucc<strong>in</strong>ate has been studied<br />
(9) <strong>in</strong> alkal<strong>in</strong>e aqueous solution. Several methods<br />
have been described for determ<strong>in</strong><strong>in</strong>g hydrocortisone<br />
<strong>and</strong> hydrocortisone succ<strong>in</strong>ate <strong>in</strong> plasma; however,<br />
<strong>the</strong> widely used liquid chromatography (HPLC) <strong>and</strong>