Laboratory Monitoring of Warfarin Therapy - Pathology
Laboratory Monitoring of Warfarin Therapy - Pathology
Laboratory Monitoring of Warfarin Therapy - Pathology
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Laboratory</strong> <strong>Monitoring</strong><br />
<strong>of</strong> <strong>Warfarin</strong> <strong>Therapy</strong><br />
David Williams, M.D., Ph.D.<br />
Roger S. Riley, M.D., Ph.D.<br />
Ann Tidwell, M.T. (ASCP) SH
<strong>Warfarin</strong> <strong>Monitoring</strong><br />
Crystalline warfarin sodium (Coumadin,<br />
Panwarfin, S<strong>of</strong>arin, Coufarin, Athrombin-K) is<br />
the most widely used oral anticoagulant in<br />
the world. <strong>Warfarin</strong> interferes with the hepatic<br />
synthesis <strong>of</strong> the vitamin-K dependent<br />
coagulation factors by interfering with the<br />
vitamin K cycle. <strong>Laboratory</strong> monitoring <strong>of</strong><br />
warfarin therapy is mandatory, since the<br />
agent has a relatively narrow therapeutic<br />
range. The therapeutic efficacy is <strong>of</strong> warfarin<br />
is reduced with an inadequate dose, while there<br />
is a serious risk <strong>of</strong> bleeding with an excess<br />
amount <strong>of</strong> the drug. In addition, the pharmacokinetics<br />
<strong>of</strong> warfarin are significantly influenced<br />
by the diet, other medications, and<br />
many other factors. During the past two decades,<br />
several developments in laboratory science<br />
have helped the clinician to ensure a<br />
proper dosage intensity that decreases the<br />
risk <strong>of</strong> bleeding while maintaining therapeutic<br />
efficacy. These developments include improved<br />
laboratory reagents and instrumentation,<br />
as well as a standardized method for reporting<br />
the prothrombin time (i.e., International<br />
Normalized Ratio, INR). However, a comprehensive<br />
system <strong>of</strong> quality assurance is still<br />
necessary for optimal patient care.<br />
It is imparative that physicians monitoring<br />
warfarinized patients must understand the biological<br />
effect and pharmacokinetics <strong>of</strong> warfarin,<br />
its interaction with other medications, and the<br />
factors affecting the prothrombin time/INR assay,<br />
including the recently discovered impact <strong>of</strong><br />
the CYP2C9 and VKORC1 genetic polymorphisms.<br />
The physician must also work with<br />
other members <strong>of</strong> the patient care team to ensure<br />
that patient identification procedures are<br />
followed and the proper type and amount <strong>of</strong><br />
specimen is submitted for laboratory testing.<br />
<strong>Warfarin</strong> is a synthetic derivative <strong>of</strong> the<br />
naturally occurring anti-coagulant dicumarol.<br />
The anti-coagulant properties <strong>of</strong> dicumarol<br />
were first observed in cattle that suffered a<br />
hemorrhagic disorder after being fed spoiled<br />
sweet clover hay during the 1920s. Karl Link<br />
identified the causative agent by 1939 and<br />
later developed warfarin as a rat poison before<br />
it was used in humans in the 1950s. However<br />
the anticoagulant mechanism <strong>of</strong> warfarin<br />
was not elucidated until twenty years<br />
later.<br />
<strong>Warfarin</strong> is a specific inhibitor <strong>of</strong> the vitamin<br />
K epoxide reductase necessary for the<br />
regeneration <strong>of</strong> vitamin K from vitamin K<br />
2,3-epoxide in vivo (Fig. 1). Vitamin K acts as<br />
a c<strong>of</strong>actor for !-glutamyl carboxylase, which<br />
carboxylates specific glutamic acid residues<br />
in vitamin K dependent proteins. The vitamin<br />
K dependent proteins include coagulation<br />
factors V, VII, IX, and X as well as protein C<br />
and protein S. This post-translational modification<br />
forms !-carboxyglutamic acid residues<br />
that chelate metal ions and allow the proteins<br />
to bind specific c<strong>of</strong>actors on phospholipid<br />
surfaces necessary for normal coagulation.<br />
The carboxylation reaction generates vitamin<br />
K 2,3-epoxide, which must be converted back<br />
to vitamin K by an epoxide reductase to<br />
maintain stores. Inhibition <strong>of</strong> vitamin K epoxide<br />
reductase reduces the availability <strong>of</strong> vitamin<br />
K, which leads to a decrease in the active<br />
forms <strong>of</strong> vitamin K dependent coagulation<br />
factors and ultimately anti-coagulation.<br />
<strong>Warfarin</strong> is currently the only FDA approved<br />
orally available anti-coagulant medication.<br />
It is rapidly absorbed from the gastrointestinal<br />
tract, reaching maximum concentration<br />
90 minutes after administration, with<br />
a half-life <strong>of</strong> 36 to 42 hours. However, the<br />
pharmacokinetics <strong>of</strong> warfarin can by highly<br />
variable. It circulates bound to albumin and is<br />
metabolized in the liver. Genetic variation in<br />
a cytochrome P450 enzyme alters the dose-<br />
<strong>Warfarin</strong> Structure, Metabolism, Pharmacokinetics 2
<strong>Warfarin</strong> <strong>Monitoring</strong><br />
O<br />
OH<br />
O<br />
O<br />
CH 3<br />
Fig. 1. The biochemistry <strong>of</strong> warfarin.<br />
(A) The molecular structure <strong>of</strong> warfarin.<br />
(B) A simplied schematic <strong>of</strong> the<br />
vitamin K cycle, showing the effect <strong>of</strong><br />
warfarin on the synthesis <strong>of</strong> vitamin-K<br />
dependent coagulation factors. In the<br />
cycle, vitamin K1 or K2 are reduced to<br />
their hydroquinone forms (KH2). A further<br />
stepwise oxidation <strong>of</strong> KH2 to vitamin<br />
K epoxide (KO) occurs, during<br />
which descarboxyprothrombin<br />
(descarboxy-II) is converted to<br />
prothrombin by carboyxlation <strong>of</strong> glutamate<br />
residues (Glu) to g-<br />
carboxyglutamate (Gla). Enzymatic<br />
reduction <strong>of</strong> the epoxide requires reduced<br />
nicotinamide adenine dinucleotide<br />
(NADH) as a c<strong>of</strong>actor and is sensitive<br />
to warfarin (Stop sign). The R<br />
sidechain on the vitamin K molecule is<br />
a 20-carbon phytyl in vitamin K1 and a<br />
5- to 65-carbon prenyl side chain in<br />
vitamin K2.<br />
response relationship as well as a number <strong>of</strong><br />
environmental, dietary, and disease related<br />
factors. A long list <strong>of</strong> commonly prescribed<br />
medications can either enhance or interfere<br />
with warfarin anti-coagulation by various<br />
mechanisms. For example, amiodarone inhibits<br />
clearance via hepatic metabolism and<br />
enhances anti-coagulation while carabamezepine,<br />
barbiturates, and rifampicin<br />
increase metabolic clearance and inhibit<br />
anti-coagulation. Interestingly, there are specific<br />
inherited mutations <strong>of</strong> factor IX that<br />
render it particularly sensitive to decreased<br />
carboxylation such that the anti-coagulation<br />
effect <strong>of</strong> warfarin is enhanced without prolonging<br />
the prothrombin time. Furthermore,<br />
dietary changes can have a pr<strong>of</strong>ound effect<br />
on anti-coagulation. Vitamin K is largely<br />
found in green vegetables such that increased<br />
intake <strong>of</strong> leafy green vegetables can<br />
provide vitamin K bypassing the need to regenerate<br />
stores with epoxide reductase.<br />
The variable pharmacokinetics <strong>of</strong> warfarin<br />
requires careful monitoring and dose<br />
adjustments to achieve a therapeutic range.<br />
Even after stabilizing within a therapeutic<br />
range, monitoring is still necessary since so<br />
<strong>Warfarin</strong> <strong>Monitoring</strong> 3
<strong>Warfarin</strong> <strong>Monitoring</strong><br />
many environmental and dietary factors can<br />
change the dose-response. Measuring the<br />
prothrombin time (PT) remains the primary<br />
mechanism by which warfarin anticoagulation<br />
is monitored. Prolongation <strong>of</strong><br />
the PT, however, depends on the responsiveness<br />
<strong>of</strong> the thromboplastin used to initiate<br />
coagulation. Hence, the WHO has currently<br />
established and international standard by<br />
which manufacturers or individual labs can<br />
determine an international sensitivity index<br />
(ISI) for each lot <strong>of</strong> thromboplastin. A therapeutic<br />
range <strong>of</strong> PT is then defined by the international<br />
normalized ratio (INR), which is<br />
the ratio between the patients PT to the mean<br />
normal PT for the lab raised to the power <strong>of</strong><br />
the ISI:<br />
Fig. 2. A nomogram for obtaining<br />
an INR from a measured<br />
prothrombin ratio (i.e., ratio <strong>of</strong><br />
patient PT/mean normal PT). The<br />
ISI is provided by the manufacturer<br />
<strong>of</strong> each lot <strong>of</strong> prothrombin<br />
reagent (thromboplastin). In the<br />
example shown, an INR <strong>of</strong> 3.0 is<br />
obtained from a plasma sample<br />
with a PTR <strong>of</strong> 2.0 if the ISI is 1.6.<br />
INR = (Patient PT/Mean Normal PT) ISI<br />
where the “mean normal PT” is the geometric<br />
mean prothrombin time for the laboratory.<br />
The ISI is a correction factor, specific for each<br />
lot <strong>of</strong> laboratory prothrombin reagent, that<br />
correlates the activity <strong>of</strong> the reagent to a<br />
standard maintained by the World Health Organization.<br />
A smaller ISI reflects a more sensitive<br />
thromboplastin reagent such that a<br />
more prolonged PT will be observed for the<br />
same therapeutic effect.<br />
In most medical institutions, the INR is<br />
automatatically calculated by the laboratory<br />
information system. The INR can also be calculated<br />
from the above formula or derived<br />
from an INR nomogram (Fig. 2).<br />
The dosing <strong>of</strong> warfarin is driven by<br />
monitoring the prothrombin time to achieve<br />
a PT in the appropriate therapeutic range.<br />
Typically 5 to 10 mg <strong>of</strong> warfarin is administered<br />
daily with daily monitoring <strong>of</strong> the INR.<br />
The dose is changed gradually until therapeutic<br />
and then the INR is followed on a regular<br />
but less frequent basis. Adjustments in dosage<br />
should be based on the weekly dose since<br />
<strong>Warfarin</strong> <strong>Monitoring</strong> 4
<strong>Warfarin</strong> <strong>Monitoring</strong><br />
warfarin has a long half-life. For most<br />
indications the INR should be maintained<br />
between 2.0 to 3.0. Exceptions include<br />
anticoagulation in patients with<br />
mechanical prosthetic heart valves and<br />
patients with the antiphospholipid antibody<br />
syndrome, in which the INR is usually<br />
kept between 2.5 and 3.5. However, it<br />
must be remembered that the INR was<br />
developed for deep venous thrombosis<br />
prophylaxis and may not be the most appropriate<br />
monitoring mechanism for<br />
other situations. Furthermore, inhibitors<br />
such as lupus anticoagulants can prolong<br />
the PT obscuring the appropriate therapeutic<br />
range such that it may be necessary<br />
to use alternative assays such as the<br />
direct quantitation <strong>of</strong> the plasma warfarin<br />
concentration.<br />
Fig. 3. Recommended therapeutic<br />
ranges for oral anticoagulant therapy.<br />
Most recent recommendations<br />
<strong>of</strong> the American College <strong>of</strong> chest<br />
Physicians and the National Heart,<br />
Lung, and Blood institute. From<br />
Ansell et al. 2004.<br />
As with all anticoagulant medications,<br />
the primary risk for warfarin therapy is<br />
hemorrhage. The risk is greater the<br />
higher the INR, highlighting the importance<br />
<strong>of</strong> regular monitoring. The risk <strong>of</strong><br />
a major bleed is 1 to 2 percent per year<br />
with only a 0.1 to 0.5 percent per year<br />
risk <strong>of</strong> intracranial hemorrhage. If a pa-<br />
<strong>Warfarin</strong> Risks & Side Effects 5
<strong>Warfarin</strong> <strong>Monitoring</strong><br />
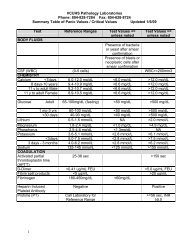
Table I<br />
Clinical Management <strong>of</strong> the Supratherapeutic INR*<br />
*Data from Ansell, 2004 and other sources. The purpose <strong>of</strong> this table is to convey general guidelines for the treatment <strong>of</strong> a<br />
supratherapeutic INR. Clinical judgment is required for the individual patient.<br />
<strong>Warfarin</strong> Risks and Side Effects 6
<strong>Warfarin</strong> <strong>Monitoring</strong><br />
tient is actively bleeding and the INR needs to<br />
be corrected acutely, fresh frozen plasma can<br />
be administered with vitamin K and in extreme<br />
12cases prothrombin complex concentrates<br />
can be given. When a patient is found<br />
to have a markedly elevated PT without active<br />
bleeding, a more conservative approach is<br />
warranted such as simply skipping dosages<br />
until the INR returns to the appropriate range<br />
(Table I).<br />
<strong>Warfarin</strong>-induced skin necrosis is a rare<br />
but significant side effect. It follows administration<br />
by one to ten days, is heralded by<br />
parasthesia and an erythematous flush, which<br />
is followed by petechiae and hemorrhage<br />
which leads to skin necrosis. Skin necrosis is<br />
most commonly associated with proctein C<br />
deficiency and occasionally protein S deficiency.<br />
Protein C has a very short half-life<br />
and a rapid decline in activity from over 50%<br />
to less than 5% apparently initiates the development<br />
<strong>of</strong> skin necrosis in these patients.<br />
Therefore, screening for protein C and S deficiencies<br />
in patients with deep venous thrombosis<br />
may help to identify those with an increased<br />
risk <strong>of</strong> skin necrosis.<br />
Ansell, J. et al. The Pharmacology and Management<br />
<strong>of</strong> the Vitamin K Antagonists: The<br />
Seventh ACCP Conference on Antithrombotic<br />
and Thrombolytic <strong>Therapy</strong>. Chest 126: 204-<br />
233, 2004.<br />
DeLoughery, T.G. “Oral anticoagulants” in<br />
Disorders <strong>of</strong> Hemostasis and Thrombosis, a<br />
Clinical Guide, Second Edition. Eds Goodnight,<br />
S.H. Jr. & Hathaway, W.E.. The McGraw-<br />
Hill Companies, New York, pp. 553-566, 2001.<br />
Fairweather, R.B., Ansell, J., van den Besselaar,<br />
A.M.H.P., Brandt, J.T., Bussey, H.I., Poller, L.,<br />
Triplett, D.A. & White, R.H. College <strong>of</strong> American<br />
Pathologists Conference XXXI on laboratory<br />
monitoring <strong>of</strong> anticoagulant therapy.<br />
<strong>Laboratory</strong> monitoring <strong>of</strong> oral anticoagulant<br />
therapy. Arch. Pathol. Lab. Med. 122:768-781,<br />
1998.<br />
Gage, B.F. et al. Management and dosing <strong>of</strong><br />
warfarin therapy. Am. J. Med. 109:481– 488,<br />
2000.<br />
Hirsh, J. Oral anticoagulants: Mechanism <strong>of</strong><br />
action, clinical effectiveness, and optimal<br />
therapeutic range. Chest 119: 8S-21S, 2001.<br />
Jaffer, A.K. et al. Low–Molecular-Weight-<br />
Heparins as Periprocedural Anticoagulation<br />
for Patients on Long-Term <strong>Warfarin</strong> <strong>Therapy</strong>:<br />
A Standardized Bridging <strong>Therapy</strong> Protocol. J.<br />
Thromb. Thrombolysis. 20:11-16, 2005.<br />
Mueller, R.L. First-generation agents: aspirin,<br />
heparin and coumarins. Best Pract. Res. Clin.<br />
Haematol. 17:23-53, 2004.<br />
Riley, R.S., Fisher, L.M., and Rowe, D. Clinical<br />
utilization <strong>of</strong> the International Normalized<br />
Ratio (INR). J. Clin. Lab. Anal. 14:101-114, 2000.<br />
Sconce, E.A. The impact <strong>of</strong> CYP2C9 and<br />
VKORC1 genetic polymorphism and patient<br />
characteristics upon warfarin dose requirements:<br />
proposal for a new dosing regimen.<br />
Blood. 106:2329-2333, 2005.<br />
Tie, J.K., Nicchitta, C., von Heijne, G. & Stafford,<br />
D.W. Membrane topology mapping <strong>of</strong><br />
vitamin K epoxide reductase by in vitro<br />
translation/cotranslocation. J. Biol. Chem. 280:<br />
16410-16416, 2005.<br />
Wallin, R. and Hutson, S.M. <strong>Warfarin</strong> and the<br />
Vitamin K-Dependent !-Carboxylation System.<br />
Trends Mol. Med. 10: 299-302, 2004.<br />
References 7