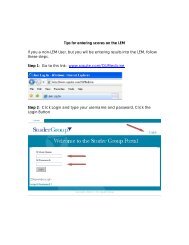
- Page 1 and 2:
Test Catalog OU MEDICAL CENTER Labo
- Page 3 and 4:
MINIMUM VOLUME: 20 mL TEST AVAILABI
- Page 5 and 6:
OTHER NAMES: Serotonin Metabolite,
- Page 7 and 8:
REFERENCE RANGE: < 0.62 mg/g METHOD
- Page 9 and 10:
Aciduria, Mitochondrial Acetoacetyl
- Page 11 and 12:
MINIMUM VOLUME: 0.3 mL (this volume
- Page 13 and 14:
TUBE OR CONTAINER: Red or Lavender
- Page 15 and 16:
REFLEX TEST INFORMATION: CPT: 84311
- Page 17 and 18:
REFERENCE RANGE: Negative: OTHER NA
- Page 19 and 20:
TEST AVAILABILITY: Daily TURNAROUND
- Page 21 and 22:
[AL] Aluminum Level SPECIMEN REQUIR
- Page 23 and 24:
SPECIMEN REQUIRED: Refrigerated bod
- Page 25 and 26:
3gm/sodium/day). SPECIMEN VOLUME: 0
- Page 27 and 28:
MINIMUM VOLUME: 0.5 mL TEST AVAILAB
- Page 29 and 30:
[ALLPOTWH] ALLERGEN POTATO WHITE [A
- Page 31 and 32:
TURNAROUND TIME: 2-9 days REFERENCE
- Page 33 and 34:
[ALU] ALUMINUM URINE SPECIMEN REQUI
- Page 35 and 36:
TUBE OR CONTAINER: Light Green (hep
- Page 37 and 38:
cirrhosis, hepatic failure, acute a
- Page 39 and 40:
TURNAROUND TIME: 8 hours REFERENCE
- Page 41 and 42:
CPT: 86038 [ANAFL] Body Fluid Anti-
- Page 43 and 44:
METHODOLOGY: Radioimmunoassay (RIA)
- Page 45 and 46:
peripheral tissues to the liver whe
- Page 47 and 48:
through Thursday only SPECIMEN VOLU
- Page 49 and 50:
METHODOLOGY: Inductively Coupled Pl
- Page 51 and 52:
TURNAROUND TIME: 4-5 days METHODOLO
- Page 53 and 54:
Testing specimen: 2 transport tubes
- Page 55 and 56:
SPECIMEN VOLUME: 10 mL (2 tubes) MI
- Page 57 and 58:
TURNAROUND TIME: 3 days REFERENCE R
- Page 59 and 60:
SPECIMEN REQUIRED: Whole blood at r
- Page 61 and 62:
METHODOLOGY: Flow Cytometry CLINICA
- Page 63 and 64:
TURNAROUND TIME: 10-14 days REFEREN
- Page 65 and 66:
CLINICAL USE: Detection of spinal f
- Page 67 and 68:
[BILDT] BILIRUBIN DIRECT AND TOTAL
- Page 69 and 70:
[BIS] Bismuth Level SPECIMEN REQUIR
- Page 71 and 72:
SPECIMEN VOLUME: 20 mL MINIMUM VOLU
- Page 73 and 74:
CPT: 82544; 83788; 84378 [BOP] Bloo
- Page 75 and 76:
[BRUCM] Brucella abortus IgM Antibo
- Page 77 and 78:
[BUNFL] Body Fluid Urea Nitrogen SP
- Page 79 and 80:
some malignancies CPT: 86332 [C3FLD
- Page 81 and 82:
therapy CPT: 86300 [CA15-3FLD] Body
- Page 83 and 84:
CPT: 83891; 83898 x 23; 83904 x 23;
- Page 85 and 86:
Spin in refrigerated centrifuge; se
- Page 87 and 88:
REFERENCE RANGE: MONO-OLIGOSACCHARI
- Page 89 and 90:
CLINICAL USE: Evaluate toxicity; mo
- Page 91 and 92:
[CATALDIS] CATALASE DISTRIBUTION FI
- Page 93 and 94:
SPECIAL HANDLING: Adjust pH to < 2
- Page 95 and 96:
CPT: 83891; 83892 x 2; 83894 x 2 ;8
- Page 97 and 98:
SPECIMEN REQUIRED: Whole blood at r
- Page 99 and 100:
stool specimen in a tightly sealed
- Page 101 and 102:
MINIMUM VOLUME: 0.5 mL (pediatric)
- Page 103 and 104:
SPECIMEN VOLUME: 1 mL MINIMUM VOLUM
- Page 105 and 106:
TEST AVAILABILITY: Daily TURNAROUND
- Page 107 and 108:
SPECIMEN VOLUME: 10 mL MINIMUM VOLU
- Page 109 and 110:
MINIMUM VOLUME: 0.5 mL (0.25-pediat
- Page 111 and 112:
METHODOLOGY: Micro-Immunofluorescen
- Page 113 and 114:
CPT: 82542 [CHOLIN] Serum Cholinest
- Page 115 and 116:
TUBE OR CONTAINER: Sterile containe
- Page 117 and 118:
REFERENCE RANGE: By report METHODOL
- Page 119 and 120:
SPECIMEN VOLUME: 0.2 mL MINIMUM VOL
- Page 121 and 122:
SPECIAL HANDLING: Acidify urine to
- Page 123 and 124:
SPECIMEN VOLUME: 1 mL MINIMUM VOLUM
- Page 125 and 126:
TUBE OR CONTAINER: Red or Dark Gree
- Page 127 and 128:
[CLTN2] CITRULLINEMIA TYPE 2(SLC25A
- Page 129 and 130:
TURN-AROUND-TIME: 14-21 days METHOD
- Page 131 and 132:
SPECIMEN REQUIRED: Serum at room te
- Page 133 and 134:
CPT: 83018 [COCCIAB] Coccidioides A
- Page 135 and 136:
-Saldino achondrogenesis, Kniest dy
- Page 137 and 138:
REFERENCE RANGE: IgG: Negative: < 1
- Page 139 and 140:
TEST AVAILABILITY: Daily METHODOLOG
- Page 141 and 142:
TUBE OR CONTAINER: EDTA (lavender)
- Page 143 and 144:
hereditary; Evaluation of progressi
- Page 145 and 146:
TURNAROUND TIME: OTHER NAMES: CoQ10
- Page 147 and 148:
TUBE OR CONTAINER: Gold or Light Gr
- Page 149 and 150:
METHODOLOGY: Complement Fixation (C
- Page 151 and 152:
SPECIMEN VOLUME: 5 mL MINIMUM VOLUM
- Page 153 and 154:
SPECIMEN VOLUME: 2 mL MINIMUM VOLUM
- Page 155 and 156:
MINIMUM VOLUME: 0.5 mL (serum) 0.5
- Page 157 and 158:
TEST AVAILABILITY: Daily TURNAROUND
- Page 159 and 160:
or sterile tube SPECIAL HANDLING: S
- Page 161 and 162:
analysis targeting 1.8 million copy
- Page 163 and 164:
TUBE OR CONTAINER: Para-Pak C&S ora
- Page 165 and 166:
TUBE OR CONTAINER: Sterile containe
- Page 167 and 168:
facility. A minimum of two sets of
- Page 169 and 170:
MINIMUM VOLUME: 5 mL TEST AVAILABIL
- Page 171 and 172:
SPECIMEN REQUIRED: Sputum or swab C
- Page 173 and 174:
SPECIMEN VOLUME: 10 mL MINIMUM VOLU
- Page 175 and 176:
CLINICAL USE: Isolate and identify
- Page 177 and 178:
SPECIAL HANDLING: Indicate source;
- Page 179 and 180:
TEST AVAILABILITY: Referral to Focu
- Page 181 and 182:
[CULSINAA] Sinus A/An Cult & Gram S
- Page 183 and 184:
screen for colonization by Group B
- Page 185 and 186:
ORDERING MODULE: MIC TEST INCLUDES:
- Page 187 and 188:
METHODOLOGY: Culture CLINICAL USE:
- Page 189 and 190:
[CYAN] Cyanide SPECIMEN REQUIRED: R
- Page 191 and 192:
[CYP2C219] CLOPIDOGREL CYPC219 GENO
- Page 193 and 194:
REFERENCE RANGE: < 0.2 nmoles half
- Page 195 and 196:
CPT: 83890; 83891, 83894; 83898 x 8
- Page 197 and 198:
SPECIMEN VOLUME: 3 mL MINIMUM VOLUM
- Page 199 and 200:
CLINICAL USE: Monitor therapeutic l
- Page 201 and 202:
MINIMUM VOLUME: 0.6 mL TEST AVAILAB
- Page 203 and 204:
[DNADAB] Anti-Double Strand DNA Ant
- Page 205 and 206:
(LC/MS-MS) CLINICAL USE: Evaluate t
- Page 207 and 208:
TEST AVAILABILITY: Monday-Thursday
- Page 209 and 210:
TURNAROUND TIME: 1-4 days REFERENCE
- Page 211 and 212:
MINIMUM VOLUME: 1 mL TEST AVAILABIL
- Page 213 and 214:
[EBVEACSF] Epstein-Barr Early Antig
- Page 215 and 216:
[EDAR] ECTODERMAL DYSPL (EDAR GENE)
- Page 217 and 218:
[EHISAB] Entamoeba histolytica Anti
- Page 219 and 220:
(A, F, S, and C), evaluate hemolyti
- Page 221 and 222:
METHODOLOGY: Indirect fluorescent a
- Page 223 and 224:
(PCR-RT) CLINICAL USE: Detection of
- Page 225 and 226:
[ESTRI] Estriol Level SPECIMEN REQU
- Page 227 and 228:
CPT: 82690 [ETHGLY] Ethylene Glycol
- Page 229 and 230:
TEST AVAILABILITY: Daily TURNAROUND
- Page 231 and 232:
SPECIMEN REQUIRED: Frozen plasma TU
- Page 233 and 234:
TUBE OR CONTAINER: Blue (Citrate) S
- Page 235 and 236:
estriction enzyme digestion and gel
- Page 237 and 238:
TURNAROUND TIME: 24 hours REFERENCE
- Page 239 and 240:
[FAPDD] FAP DUPLICATION/DELETION SP
- Page 241 and 242:
efore specimen is drawn SPECIMEN VO
- Page 243 and 244: SPECIMEN REQUIRED: Amniotic fluid T
- Page 245 and 246: SPECIMEN REQUIRED: Whole blood at r
- Page 247 and 248: [FKTN] FUKUTIN (FKTN) GENE SEQUENCI
- Page 249 and 250: [FLT3] FLT3 INTERNAL TANDEM DUP SPE
- Page 251 and 252: OTHER NAMES: Prolixin; Permitil MET
- Page 253 and 254: METHODOLOGY: Electrochemiluminescen
- Page 255 and 256: TURNAROUND TIME: 4 weeks OTHER NAME
- Page 257 and 258: and fructose-1,6 diphosphatase defi
- Page 259 and 260: [FTACSF] CSF Treponema pallidum Ab
- Page 261 and 262: [FUNSENS] FUNGUS SENSITIVITY TEST O
- Page 263 and 264: TEST AVAILABILITY: Daily TURNAROUND
- Page 265 and 266: [GABRG2] g-AMINOBUTYRIC RECEPT GAMM
- Page 267 and 268: REFERENCE RANGE: METHODOLOGY: Spect
- Page 269 and 270: [GAST] Gastrin SPECIMEN REQUIRED: F
- Page 271 and 272: CPT: 83891; 83894; 83898 x 25; 8390
- Page 273 and 274: OTHER NAMES: HGH; Human Growth Horm
- Page 275 and 276: REFERENCE RANGE: IgA: Negative: 0-1
- Page 277 and 278: CPT: 82943 [GLUCSF] CSF Glucose SPE
- Page 279 and 280: SPECIMEN REQUIRED: Frozen serum or
- Page 281 and 282: TURNAROUND TIME: 2-6 days METHODOLO
- Page 283 and 284: SPECIMEN VOLUME: 1 mL MINIMUM VOLUM
- Page 285 and 286: SPECIMEN VOLUME: 5 mL each tube MIN
- Page 287 and 288: SPECIMEN REQUIRED: Whole blood; Liv
- Page 289 and 290: food and liquids (even water) durin
- Page 291 and 292: MINIMUM VOLUME: 0.5 mL TEST AVAILAB
- Page 293: SPECIMEN REQUIRED: Refrigerated ser
- Page 297 and 298: TEST AVAILABILITY: Daily TURNAROUND
- Page 299 and 300: TUBE OR CONTAINER: Red or Gold SPEC
- Page 301 and 302: METHODOLOGY: Atomic absorption spec
- Page 303 and 304: [HEMAINV] HEMOPHILIA A INVERSION (D
- Page 305 and 306: TEST AVAILABILITY: Daily TURNAROUND
- Page 307 and 308: coagulant to blood ratio; Invert ge
- Page 309 and 310: TURNAROUND TIME: 7-10 days REFERENC
- Page 311 and 312: OTHER NAMES: Free Hemoglobin; Hgb S
- Page 313 and 314: TURNAROUND TIME: 3-4 weeks REFERENC
- Page 315 and 316: 1 mL (CSF-frozen) MINIMUM VOLUME: 3
- Page 317 and 318: MINIMUM VOLUME: 0.5 mL TEST INCLUDE
- Page 319 and 320: Heparin-Induced Thrombocytopenia Ab
- Page 321 and 322: REFLEX TEST INFORMATION: CLINICAL U
- Page 323 and 324: SPECIMEN VOLUME: 2 mL MINIMUM VOLUM
- Page 325 and 326: [HOMOCUP] UR HOMOCYSTEINE PANEL SPE
- Page 327 and 328: TUBE OR CONTAINER: Lavender (EDTA)
- Page 329 and 330: SPECIMEN VOLUME: 1 mL MINIMUM VOLUM
- Page 331 and 332: SPECIMEN VOLUME: 1 mL MINIMUM VOLUM
- Page 333 and 334: CPT: 82570; 83150 [HWS] ECTODERMAL
- Page 335 and 336: METHODOLOGY: Polymerase Chain React
- Page 337 and 338: [IDIHIVINTEG] HIV Integrase Inhibit
- Page 339 and 340: [IEPRAN] UR IMMUNOELECTROPHOR RANDO
- Page 341 and 342: performed on a lipemic sample SPECI
- Page 343 and 344: TEST AVAILABILITY: Daily TURNAROUND
- Page 345 and 346:
CPT: 82784 [IGGFLD] Body Fluid Immu
- Page 347 and 348:
TUBE OR CONTAINER: Sterile containe
- Page 349 and 350:
MINIMUM VOLUME: 0.2 mL in two separ
- Page 351 and 352:
SPECIMEN VOLUME: 1 mL MINIMUM VOLUM
- Page 353 and 354:
[INT10] Interleukin 10 (IL-10) SPEC
- Page 355 and 356:
TUBE OR CONTAINER: Red, Gold or Lav
- Page 357 and 358:
METHODOLOGY: Liquid Chromatography/
- Page 359 and 360:
OTHER NAMES: JAK2 Mutation METHODOL
- Page 361 and 362:
TEST AVAILABILITY: Daily TURNAROUND
- Page 363 and 364:
REFERENCE RANGE: No mutation detect
- Page 365 and 366:
TUBE OR CONTAINER: Lavender (EDTA)
- Page 367 and 368:
container or between two microscope
- Page 369 and 370:
[L1CAM] L1CAM MUTATION SPECIMEN REQ
- Page 371 and 372:
OTHER NAMES: HPL; Human chorionic s
- Page 373 and 374:
myelofibrosis with myeloid metaplas
- Page 375 and 376:
TUBE OR CONTAINER: Sterile containe
- Page 377 and 378:
[LDHPT] Peritoneal Fluid LDH SPECIM
- Page 379 and 380:
Separate from cells within 45 minut
- Page 381 and 382:
[LEP] Leptin SPECIMEN REQUIRED: Fro
- Page 383 and 384:
SPECIMEN REQUIRED: Whole blood at r
- Page 385 and 386:
TEST AVAILABILITY: Daily TURNAROUND
- Page 387 and 388:
[LISTAB] Listeria Antibody SPECIMEN
- Page 389 and 390:
TEST AVAILABILITY: Daily OTHER NAME
- Page 391 and 392:
[LQTS] LONG QT SYNDROME SPECIMEN RE
- Page 393 and 394:
TUBE OR CONTAINER: Red or Gold SPEC
- Page 395 and 396:
SPECIAL HANDLING: Specimen must arr
- Page 397 and 398:
[MACAMY] MACROAMYLASE SPECIMEN REQU
- Page 399 and 400:
TEST AVAILABILITY: Daily TURNAROUND
- Page 401 and 402:
loci CLINICAL USE: Ensure accurate
- Page 403 and 404:
TURNAROUND TIME: 4-6 weeks OTHER NA
- Page 405 and 406:
1.11 AU or greater: Positive METHOD
- Page 407 and 408:
Do NOT collect specimen in gel barr
- Page 409 and 410:
SPECIMEN VOLUME: 1 mL MINIMUM VOLUM
- Page 411 and 412:
CPT: 82131 [METHIOU] Urine Methioni
- Page 413 and 414:
SPECIMEN REQUIRED: Refrigerated uri
- Page 415 and 416:
lock MINIMUM VOLUME: Two unstained
- Page 417 and 418:
[MICMISC] Miscellaneous Microbiolog
- Page 419 and 420:
[MICSPOR] Microsporidium Smear SPEC
- Page 421 and 422:
SPECIMEN REQUIRED: Whole blood at r
- Page 423 and 424:
REFERENCE RANGE: Negative METHODOLO
- Page 425 and 426:
CLINICAL USE: Detects sequence vari
- Page 427 and 428:
TEST AVAILABILITY: Daily TURNAROUND
- Page 429 and 430:
CLINICAL USE: Determine immunity to
- Page 431 and 432:
SPECIMEN REQUIRED: Serum at room te
- Page 433 and 434:
METHODOLOGY: Polymerase Chain React
- Page 435 and 436:
OTHER NAMES: Hypertrophic Cardiomyo
- Page 437 and 438:
secretion or other diseases involvi
- Page 439 and 440:
SPECIMEN REQUIRED: Liquid stool TUB
- Page 441 and 442:
METHODOLOGY: Polyermase Chain React
- Page 443 and 444:
SPECIMEN REQUIRED: Frozen plasma TU
- Page 445 and 446:
TEST AVAILABILITY: Monday - Thursda
- Page 447 and 448:
TUBE OR CONTAINER: Dark green (hepa
- Page 449 and 450:
[NOMAR] Nomarski Prep [NORDIAZ] Nor
- Page 451 and 452:
SPECIMEN REQUIRED: Refrigerated who
- Page 453 and 454:
OTHER NAMES: N-Telopeptide crosslin
- Page 455 and 456:
[OCCBLDG] Gastric Occult Blood SPEC
- Page 457 and 458:
TEST INCLUDES: CSF and serum oligoc
- Page 459 and 460:
[ORGQNT] ORGANIC ACIDS QUANTITATIVE
- Page 461 and 462:
MINIMUM VOLUME: 1 mL TEST AVAILABIL
- Page 463 and 464:
(LC/MS/MS) CLINICAL USE: Therapeuti
- Page 465 and 466:
TEST AVAILABILITY: Daily REFERENCE
- Page 467 and 468:
TEST INCLUDES: Anti-Neuronal Nuclea
- Page 469 and 470:
[PARVPCR] Parvovirus B19 DNA (PCR)
- Page 471 and 472:
cannot be reliably evaluated SPECIM
- Page 473 and 474:
[PDHA1] PYRUV DEHYDROGENASE DEFICIE
- Page 475 and 476:
MINIMUM VOLUME: 1.5 mL (this volume
- Page 477 and 478:
testing) TEST AVAILABILITY: Daily T
- Page 479 and 480:
[PEDIGFBP1] Insulin-like GF Binding
- Page 481 and 482:
TUBE OR CONTAINER: Red, Gold, Laven
- Page 483 and 484:
SPECIMEN REQUIRED: Fibroblasts at r
- Page 485 and 486:
SPECIMEN VOLUME: 5 mL MINIMUM VOLUM
- Page 487 and 488:
TEST AVAILABILITY: Daily TURNAROUND
- Page 489 and 490:
MINIMUM VOLUME: 1 mL TEST AVAILABIL
- Page 491 and 492:
TURNAROUND TIME: 8 hours REFERENCE
- Page 493 and 494:
[PIN] Pinworm Exam SPECIMEN REQUIRE
- Page 495 and 496:
TUBE OR CONTAINER: Blue (Citrate) S
- Page 497 and 498:
SPECIMEN REQUIRED: Whole blood bloo
- Page 499 and 500:
REFERENCE RANGE: Normal - no duplic
- Page 501 and 502:
REFERENCE RANGE: Erythritol: 55 - 1
- Page 503 and 504:
fluorometric detection CLINICAL USE
- Page 505 and 506:
OTHER NAMES: Noxafil METHODOLOGY: H
- Page 507 and 508:
[PRIM] PRIMIDONE/PHENOBARBITAL SPEC
- Page 509 and 510:
TUBE OR CONTAINER: Light Green (hep
- Page 511 and 512:
REFERENCE RANGE: FEP, blood: enviro
- Page 513 and 514:
REFERENCE RANGE: None established M
- Page 515 and 516:
[PS1] ADMARK PS-1 ANALYSIS SPECIMEN
- Page 517 and 518:
times to prevent clotting Send to l
- Page 519 and 520:
Send to lab immediately or centrifu
- Page 521 and 522:
Testing volume: 1 mL of PLASMA TEST
- Page 523 and 524:
TURNAROUND TIME: 3 weeks METHODOLOG
- Page 525 and 526:
interferon-gamma production with de
- Page 527 and 528:
MINIMUM VOLUME: 0.1 mL TEST AVAILAB
- Page 529 and 530:
MINIMUM VOLUME: 1 mL (this volume d
- Page 531 and 532:
TUBE OR CONTAINER: Lavender (EDTA)
- Page 533 and 534:
SPECIMEN REQUIRED: Whole blood at r
- Page 535 and 536:
[RIBCSF] Ribosomal P Protein Antibo
- Page 537 and 538:
no additional charge CLINICAL USE:
- Page 539 and 540:
OTHER NAMES: Measles antibodies MET
- Page 541 and 542:
MINIMUM VOLUME: 2 mL (whole blood)
- Page 543 and 544:
SPECIMEN REQUIRED: Fibroblasts at r
- Page 545 and 546:
MINIMUM VOLUME: 10 mL TEST AVAILABI
- Page 547 and 548:
VOLUME: 2 flasks METHODOLOGY: Immun
- Page 549 and 550:
SPECIMEN VOLUME: 10 mL MINIMUM VOLU
- Page 551 and 552:
REFERENCE RANGE: Environmental expo
- Page 553 and 554:
TURNAROUND TIME: 6-12 days METHODOL
- Page 555 and 556:
same day as collected SPECIMEN VOLU
- Page 557 and 558:
(testing) TEST INCLUDES: IgG antibo
- Page 559 and 560:
METHODOLOGY: Indirect fluorescent a
- Page 561 and 562:
[SMTHAB] Anti-Smooth Muscle Antibod
- Page 563 and 564:
SPECIMEN REQUIRED: Whole blood at r
- Page 565 and 566:
TUBE OR CONTAINER: Transfer tube SP
- Page 567 and 568:
METHODOLOGY: Flow cytometry [STERAB
- Page 569 and 570:
SPECIMEN VOLUME: 300 mL (2 aliquots
- Page 571 and 572:
[STRONGG] Strongyloides IgG Antibod
- Page 573 and 574:
[SUCCINYL] Urine Succinylacetone SP
- Page 575 and 576:
Glyburide, Tolazamide, Tolbutamide
- Page 577 and 578:
CPT: 84481 [T3REV] Reverse Triiodot
- Page 579 and 580:
[TACRO] Tacrolimus (Prograf) Level
- Page 581 and 582:
euthyroid individuals who have norm
- Page 583 and 584:
TURNAROUND TIME: 24 hours METHODOLO
- Page 585 and 586:
REFERENCE RANGE: Age specific METHO
- Page 587 and 588:
TEST AVAILABILITY: Monday-Thursday
- Page 589 and 590:
TUBE OR CONTAINER: Red or Dark gree
- Page 591 and 592:
Send list of other antibiotics pati
- Page 593 and 594:
METHODOLOGY: Polymerase chain react
- Page 595 and 596:
TEST AVAILABILITY: Daily TURNAROUND
- Page 597 and 598:
[TOXOA] Toxoplasma IgA Antibody SPE
- Page 599 and 600:
SPECIMEN VOLUME: 5 mL MINIMUM VOLUM
- Page 601 and 602:
[TRANSF] Transferrin SPECIMEN REQUI
- Page 603 and 604:
REFERENCE RANGE: < 5 pg/mL METHODOL
- Page 605 and 606:
REFERENCE RANGE: None established M
- Page 607 and 608:
METHODOLOGY: Chemiluminescence CLIN
- Page 609 and 610:
SPECIMEN VOLUME: 2 mL MINIMUM VOLUM
- Page 611 and 612:
TURNAROUND TIME: 2-4 Days METHODOLO
- Page 613 and 614:
METHODOLOGY: Enzyme-linked immunoso
- Page 615 and 616:
REFLEX TEST INFORMATION: A urine cu
- Page 617 and 618:
TEST AVAILABILITY: Monday-Thursday
- Page 619 and 620:
[UR FLUORIDE] UR FLUORIDE SPECIMEN
- Page 621 and 622:
TURNAROUND TIME: 8 hours REFERENCE
- Page 623 and 624:
MINIMUM VOLUME: 30 mL TEST INCLUDES
- Page 625 and 626:
METHODOLOGY: Direct Fluorescent ant
- Page 627 and 628:
TEST AVAILABILITY: Monday-Saturday
- Page 629 and 630:
SPECIMEN REQUIRED: Serum TUBE OR CO
- Page 631 and 632:
CLINICAL INFORMATION: Aid in the di
- Page 633 and 634:
will be processed but not stained a
- Page 635 and 636:
quantitative test at an additional
- Page 637 and 638:
REFERENCE RANGE: Negative METHODOLO
- Page 639 and 640:
3. Place the tubing in the nasophar
- Page 641 and 642:
the freshest lesion or vesicle (dew
- Page 643 and 644:
SPECIMEN REQUIRED: Refrigerated spe
- Page 645 and 646:
TUBE OR CONTAINER: Viral transport
- Page 647 and 648:
TURNAROUND TIME: 24 hours REFERENCE
- Page 649 and 650:
CLINICAL USE: Rapid identification
- Page 651 and 652:
5. After or before doing the wash,
- Page 653 and 654:
poor or if the FA is negative. The
- Page 655 and 656:
viral transport media tube. Refrige
- Page 657 and 658:
[VITA] Vitamin A Level SPECIMEN REQ
- Page 659 and 660:
[VITB5] Pantothenic Acid Level SPEC
- Page 661 and 662:
CLINICAL USE: Rule out vitamin D de
- Page 663 and 664:
METHODOLOGY: Liquid chromatography/
- Page 665 and 666:
5. After or before doing the wash,
- Page 667 and 668:
5. After or before doing the wash,
- Page 669 and 670:
clumps of mucous; spread the specim
- Page 671 and 672:
1. Patient should not have urinated
- Page 673 and 674:
[VPGCDNAP] N. GONORRHOEAE BY PCR SP
- Page 675 and 676:
MINIMUM VOLUME: 0.5 mL TEST INCLUDE
- Page 677 and 678:
Nasopharyngeal wash+NP instructions
- Page 679 and 680:
TEST AVAILABILLTIY: Monday-Saturday
- Page 681 and 682:
port medium or have the patient gar
- Page 683 and 684:
a good epithelial cell scraping of
- Page 685 and 686:
SPECIMEN REQUIRED: Refrigerated spe
- Page 687 and 688:
[VW2B] VON WILLEBRAND TYPE 2B BINDI
- Page 689 and 690:
SPECIAL HANDLING: Must be received
- Page 691 and 692:
TEST AVAILABILITY: Daily OTHER NAME
- Page 693 and 694:
[VZVPCR] Varicella-Zoster Virus DNA
- Page 695 and 696:
MINIMUM VOLUME: 0.5 mL TEST INCLUDE
- Page 697 and 698:
device SPECIAL HANDLING: A delay of
- Page 699 and 700:
TEST AVAILABILITY: Daily TURNAROUND
- Page 701 and 702:
Discard this sample. Collect urine
- Page 703 and 704:
TEST AVAILABILITY: Daily REFERENCE