MOM 2006 journal for pdf.pmd - University of Michigan-Flint
MOM 2006 journal for pdf.pmd - University of Michigan-Flint
MOM 2006 journal for pdf.pmd - University of Michigan-Flint
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The relative energies <strong>of</strong> the NO adsorption complexes at the HF level show little variation among<br />
all the clusters (Table 4). There seems to be an anomaly with the 5T –OH HF energies. Looking at the<br />
DFT results, complex H is not a stable structure. There<strong>for</strong>e the energy difference between complexes<br />
F and G is in the same range as the rest <strong>of</strong> the clusters. The calculated energies at the HF level<br />
indicate that complex F is the most stable and, there<strong>for</strong>e, the most likely adsorption type. The G and<br />
H complexes are relatively closer in energy. DFT energies, however, differ significantly from the HF<br />
energies. Most <strong>of</strong> the clusters still indicated complex F as the most stable, and G is significantly<br />
higher in energy than H. The substantial differences in energies at the DFT level demonstrate the<br />
importance <strong>of</strong> electron correlation in the calculations. The 5T –H cluster provides contradictory<br />
results, though. The trend continues, however, with –OH termination. This seems to indicate that<br />
hydrogen termination is not as accurate a model <strong>of</strong> zeolite as the hydroxyl terminated clusters. Given<br />
the DFT energies, complex G is clearly the least stable <strong>of</strong> the adsorption complexes. The cluster size<br />
also makes the difference in the DFT calculations. In general, the cluster models indicate that the F<br />
complex is the most stable, followed by the G complex. When the cluster size reaches 5T and 10T,<br />
the H structure can no longer be obtained. This indicates that the H structure is not a minimum on the<br />
potential energy surface. As such, this might be a reasonable initial guess <strong>for</strong> a transition state, though<br />
that is not the focus <strong>of</strong> this study.<br />
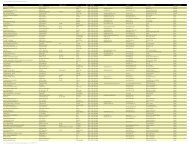
Table 4: Relative Energies ( kcal mol) <strong>of</strong> the NO adsorption complex<br />
1T 3T -H 3T -OH 5T -H 5T -OH 10T<br />
HF<br />
F 0.0 0.0 0.0 0.0 18.41 0.0<br />
G 5.52 4.27 3.94 3.86 21.96 3.06<br />
H 4.56 6.34 7.99 6.77 0.0 -<br />
DFT<br />
F 0.0 0.0 0.0 4.45 0.0 0.0<br />
G 28.49 24.89 25.06 19.46 21.39 22.33<br />
H 6.55 4.89 4.96 0.0 - -<br />
Summary<br />
The most obvious conclusion from this data is that the cluster size has a significant impact on the<br />
energies <strong>of</strong> the complexes as well as on the geometries. The H complex is a local minimum with<br />
smaller clusters but not in the large clusters. The 5T–H cluster provides results that are contrary to the<br />
general trends. This data displayed the H complex as the most stable, while F was the most stable in<br />
all other cases. Additionally, the NO 2<br />
complexes are also greatly influenced by the cluster size. The<br />
A, B, and D complexes occur with certain clusters and not with others. The reason <strong>for</strong> this is as yet<br />
unclear. In all cases, though, the DFT calculations indicate that the E complex is the most stable NO 2<br />
complex.<br />
In addition to the effects <strong>of</strong> the cluster size, the level <strong>of</strong> theory also impacts the results. Electron<br />
correlation from the DFT calculations contributes to a large increase in the relative energy <strong>of</strong> the G<br />
complex. From HF energies alone, it is not clear whether the G or H complex is more stable. DFT<br />
results, however, show a clear difference in energy between these two adsorption complexes.<br />
Complex G, then, is clearly the least stable complex, while H is not a local minimum, and thus not a<br />
stable adsorption complex, in the large clusters. The HF energies also do not clearly indicate the most<br />
Meeting <strong>of</strong> Minds <strong>2006</strong> 75