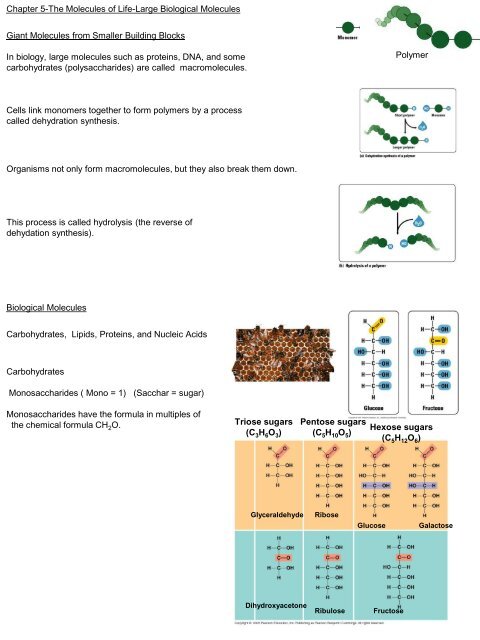
Giant Molecules from Smaller Building Blocks Cells link monomers ...
Giant Molecules from Smaller Building Blocks Cells link monomers ...
Giant Molecules from Smaller Building Blocks Cells link monomers ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 5-The <strong>Molecules</strong> of Life-Large Biological <strong>Molecules</strong><br />
<strong>Giant</strong> <strong>Molecules</strong> <strong>from</strong> <strong>Smaller</strong> <strong>Building</strong> <strong>Blocks</strong><br />
In biology, large molecules such as proteins, DNA, and some<br />
carbohydrates (polysaccharides) are called macromolecules.<br />
Polymer<br />
<strong>Cells</strong> <strong>link</strong> <strong>monomers</strong> together to form polymers by a process<br />
called dehydration synthesis.<br />
Organisms not only form macromolecules, but they also break them down.<br />
This process is called hydrolysis (the reverse of<br />
dehydation synthesis).<br />
Biological <strong>Molecules</strong><br />
Carbohydrates, Lipids, Proteins, and Nucleic Acids<br />
Carbohydrates<br />
Monosaccharides ( Mono = 1) (Sacchar = sugar)<br />
Monosaccharides have the formula in multiples of<br />
the chemical formula CH 2 O.<br />
Triose sugars<br />
(C 3 H 6 O 3 )<br />
Pentose sugars<br />
(C 5 H 10 O 5 )<br />
Hexose sugars<br />
(C 5 H 12 O 6 )<br />
Glyceraldehyde<br />
Ribose<br />
Glucose<br />
Galactose<br />
Dihydroxyacetone<br />
Ribulose<br />
Fructose
Carbohydrates<br />
Monosaccharides ( Mono = 1) (Sacchar = sugar)<br />
Disaccharides ( Di = 2) (Sacchar = sugar)<br />
Dehydration<br />
reaction in the<br />
synthesis of maltose<br />
Glucose<br />
Glucose<br />
Maltose<br />
Monomer + Monomer in the process<br />
of dehydration synthesis.<br />
Dehydration<br />
reaction in the<br />
synthesis of sucrose<br />
Glucose<br />
Fructose<br />
Sucrose<br />
Do you know what 2 plants are used in the<br />
U.S. to produce sucrose, or table sugar?<br />
Polysaccharides (poly=many) (Sacchar= sugar)<br />
Complex carbohydrates<br />
Long chains of sugar units<br />
Examples of Polysaccharides<br />
Chloroplast<br />
Starch<br />
Starch<br />
Found in roots and other plant organs.<br />
Glycogen granules<br />
Mitochondria<br />
Amylose<br />
Amylopectin<br />
Glycogen<br />
Animals store excess sugar in this polysaccharide.<br />
Glycogen is broken down in our bodies or hydrolyzed to<br />
release glucose when it is needed.
Cellulose<br />
Cellulose is the most abundant organic compound on Earth.<br />
Cellulose is also a polymer of glucose units, but<br />
the glucose <strong>monomers</strong> are <strong>link</strong>ed together in different<br />
orientation.<br />
a Glucose<br />
a and b glucose ring structures<br />
b Glucose<br />
Cell walls<br />
Cellulose microfibrils<br />
in a plant cell wall<br />
Microfibril<br />
Starch: 1–4 <strong>link</strong>age of a glucose <strong>monomers</strong>.<br />
Cellulose: 1–4 <strong>link</strong>age of b glucose <strong>monomers</strong>.<br />
b Glucose<br />
monomer<br />
Cellulose<br />
molecules<br />
•Enzymes that digest starch by hydrolyzing alpha <strong>link</strong>ages can’t<br />
hydrolyze beta <strong>link</strong>ages in cellulose<br />
Carbohydrates are hydrophilic (hydro=water; philic=loving) biological molecules.<br />
In contrast to carbohydrates and other biological molecules,lipids do not mix with water.<br />
Lipids are hydrophobic (hydro = water) (phobic=fearing)<br />
Lipids<br />
Examples of lipids are:<br />
Fats<br />
Dietary fats consist of the molecule triglyceride.<br />
The triglyceride is made of a molecule of glycerol joined with<br />
3 molecules of fatty acids.<br />
Like the hydrocarbons in gasoline, the long hydrocarbon<br />
chain of the fatty acid in a triglyceride stores a large<br />
amount of energy.<br />
A pound of fat packs more than twice as much energy as<br />
a pound of carbohydrates.
“Saturated” fats versus “unsaturated” fats<br />
he fatty acid is said to be unsaturated because it has less<br />
han the maximum number of hydrogens at the location of<br />
he double bond.<br />
Fatty acids are saturated because they are bonded to<br />
the maximum number of hydrogen atoms.<br />
Saturated fats<br />
Stearic acid<br />
- Solid at room temperature<br />
Saturated fat and fatty acid.<br />
Unsaturated fats<br />
- Usually liquid at room temperature<br />
Unsaturated fat and fatty acid.<br />
Phospholipids<br />
•In a phospholipid, two fatty acids and a phosphate group<br />
are attached to glycerol<br />
cis double bond<br />
causes bending<br />
Choline<br />
Phosphate<br />
Glycerol<br />
When phospholipids are added to water, they selfassemble<br />
into a bilayer, with the hydrophobic tails<br />
pointing toward the interior<br />
Fatty acids<br />
The structure of phospholipids results in a bilayer<br />
arrangement found in cell membranes<br />
WATER<br />
Hydrophilic<br />
head<br />
Hydrophobic<br />
tails<br />
WATER
Steroids<br />
Steroids are very different <strong>from</strong> fats in structure and function.<br />
•Cholesterol, an important steroid, is a<br />
component in animal cell membranes<br />
Chapter 5-The <strong>Molecules</strong> of Life-Large Biological <strong>Molecules</strong><br />
-Know the four biological molecules: carbohydrates, lipids, proteins, and nucleic acids<br />
-Know terms: macromolecule, polymer, monomer, dehydration synthesis, hydrolysis<br />
-Know different carbohydrates and recognize examples of each:<br />
monosaccharide, disaccharide, and polysaccharide<br />
-Recognize examples and functions of three types of polysaccharides:<br />
starch, glycogen, and cellulose<br />
-Recognize the difference of two ring forms for glucose: alpha () and beta (b)<br />
-Understand hydrophilic vs. hydrophobic<br />
-Know examples of hydrophobic and hydrophilic biomolecules<br />
-Understand the characteristics of a lipid<br />
-Know terms and structure-fat, oil, triglyceride, glycerol<br />
-Understand saturated vs. unsaturated and know examples of each<br />
-Understand the structure of a steroid