7MB pdf - American Society for Gastrointestinal Endoscopy
7MB pdf - American Society for Gastrointestinal Endoscopy
7MB pdf - American Society for Gastrointestinal Endoscopy
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Clean Freak<br />
Effective cleansing in all bowel segments, including the right colon<br />
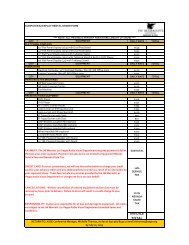
Percent of patients with NO RESIDUAL STOOL by colon segment 1 *<br />
Colon Segment<br />
SUPREP Bowel<br />
Prep Kit split-dose<br />
regimen (n=63)<br />
4-Liter Prep<br />
same-day regimen ‡<br />
(n=66) §<br />
Cecum 91% † 67%<br />
Ascending 91% † 69%<br />
Descending 92% 84%<br />
Transverse 92% 82%<br />
Sigmoid/Rectum 94% 81%<br />
*This clinical trial was not included in the product labeling. † P≤0.02 vs 4-Liter Prep. Statistically significant difference.<br />
‡<br />
Standard 4-Liter Prep (sulfate-free PEG electrolyte lavage solution).<br />
§<br />
One patient was excluded who took the preparation but refused colonoscopy. Three patients had one or more<br />
segments that could not be evaluated because the procedure was stopped <strong>for</strong> poor preparation be<strong>for</strong>e cecal intubation.<br />
SUPREP Bowel Prep Kit achieved “excellent” bowel<br />
cleansing in patients based on investigator grading 1,2<br />
• Split-dose regimens of SUPREP Bowel Prep Kit<br />
and MoviPrep ®|| were equivalent in colon<br />
cleansing 2<br />
• Significantly more patients had “excellent” preps<br />
with SUPREP Bowel Prep Kit compared to MoviPrep<br />
(63% vs 53%, respectively; P=0.043 ) 2<br />
||<br />
MoviPrep (PEG-3350, sodium sulfate, sodium chloride, potassium chloride, sodium ascorbate<br />
and ascorbic acid <strong>for</strong> oral solution) is a registered trademark of Salix Pharmaceuticals, Inc.<br />
<br />
Statistically significant difference.<br />
Important Safety In<strong>for</strong>mation<br />
SUPREP ® Bowel Prep Kit (sodium sulfate, potassium sulfate and magnesium sulfate) Oral Solution is an osmotic laxative indicated <strong>for</strong> cleansing of the colon as a preparation<br />
<strong>for</strong> colonoscopy in adults. Most common adverse reactions (>2%) are overall discom<strong>for</strong>t, abdominal distention, abdominal pain, nausea, vomiting and headache.<br />
Use is contraindicated in the following conditions: gastrointestinal (GI) obstruction, bowel<br />
per<strong>for</strong>ation, toxic colitis and toxic megacolon, gastric retention, ileus, known allergies to<br />
components of the kit. Use caution when prescribing <strong>for</strong> patients with a history of seizures,<br />
arrhythmias, impaired gag refl ex, regurgitation or aspiration, severe active ulcerative colitis,<br />
impaired renal function or patients taking medications that may affect renal function or electrolytes.<br />
Use can cause temporary elevations in uric acid. Uric acid fl uctuations in patients with gout may<br />
precipitate an acute fl are. Administration of osmotic laxative products may produce mucosal<br />
aphthous ulcerations, and there have been reports of more serious cases of ischemic colitis<br />
requiring hospitalization. Patients with impaired water handling who experience severe vomiting<br />
should be closely monitored including measurement of electrolytes. Advise all patients to hydrate<br />
adequately be<strong>for</strong>e, during, and after use. Each bottle must be diluted with water to a fi nal volume<br />
of 16 ounces and ingestion of additional water as recommended is important to patient tolerance.<br />
Please see brief summary of Prescribing In<strong>for</strong>mation on adjacent page.