efsa-opinion-chromium-food-drinking-water
efsa-opinion-chromium-food-drinking-water
efsa-opinion-chromium-food-drinking-water
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chromium in <strong>food</strong> and <strong>drinking</strong> <strong>water</strong><br />
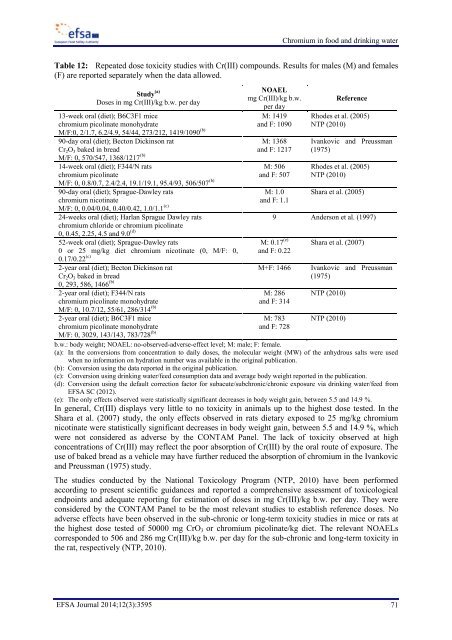
Table 12: Repeated dose toxicity studies with Cr(III) compounds. Results for males (M) and females<br />
(F) are reported separately when the data allowed.<br />
Study (a)<br />
Doses in mg Cr(III)/kg b.w. per day<br />
NOAEL<br />
mg Cr(III)/kg b.w.<br />
per day<br />
Reference<br />
M: 1419<br />
13-week oral (diet); B6C3F1 mice<br />
Rhodes et al. (2005)<br />
M/F:0, 2/1.7, 6.2/4.9, 54/44, 273/212, 1419/1090 (b)<br />
<strong>chromium</strong> picolinate monohydrate<br />
and F: 1090 NTP (2010)<br />
90-day oral (diet); Becton Dickinson rat<br />
Cr 2 O 3 baked in bread<br />
M/F: 0, 570/547, 1368/1217 (b) M: 1368<br />
and F: 1217<br />
Ivankovic and Preussman<br />
(1975)<br />
14-week oral (diet); F344/N rats<br />
<strong>chromium</strong> picolinate<br />
M/F: 0, 0.8/0.7, 2.4/2.4, 19.1/19.1, 95.4/93, 506/507 (b) M: 506<br />
and F: 507<br />
Rhodes et al. (2005)<br />
NTP (2010)<br />
90-day oral (diet); Sprague-Dawley rats<br />
M: 1.0 Shara et al. (2005)<br />
M/F: 0, 0.04/0.04, 0.40/0.42, 1.0/1.1 (c)<br />
<strong>chromium</strong> nicotinate<br />
and F: 1.1<br />
24-weeks oral (diet); Harlan Sprague Dawley rats<br />
9 Anderson et al. (1997)<br />
0, 0.45, 2.25, 4.5 and 9.0 (d)<br />
<strong>chromium</strong> chloride or <strong>chromium</strong> picolinate<br />
52-week oral (diet); Sprague-Dawley rats<br />
M: 0.17 (e) Shara et al. (2007)<br />
0.17/0.22 (c)<br />
0 or 25 mg/kg diet <strong>chromium</strong> nicotinate (0, M/F: 0, and F: 0.22<br />
2-year oral (diet); Becton Dickinson rat<br />
Cr 2 O 3 baked in bread<br />
0, 293, 586, 1466 (b) M+F: 1466 Ivankovic and Preussman<br />
(1975)<br />
2-year oral (diet); F344/N rats<br />
M: 286 NTP (2010)<br />
M/F: 0, 10.7/12, 55/61, 286/314 (b)<br />
<strong>chromium</strong> picolinate monohydrate<br />
and F: 314<br />
2-year oral (diet); B6C3F1 mice<br />
M: 783 NTP (2010)<br />
M/F: 0, 3029, 143/143, 783/728 (b)<br />
<strong>chromium</strong> picolinate monohydrate<br />
and F: 728<br />
b.w.: body weight; NOAEL: no-observed-adverse-effect level; M: male; F: female.<br />
(a): In the conversions from concentration to daily doses, the molecular weight (MW) of the anhydrous salts were used<br />
when no information on hydration number was available in the original publication.<br />
(b): Conversion using the data reported in the original publication.<br />
(c): Conversion using <strong>drinking</strong> <strong>water</strong>/feed consumption data and average body weight reported in the publication.<br />
(d): Conversion using the default correction factor for subacute/subchronic/chronic exposure via <strong>drinking</strong> <strong>water</strong>/feed from<br />
EFSA SC (2012).<br />
(e): The only effects observed were statistically significant decreases in body weight gain, between 5.5 and 14.9 %.<br />
In general, Cr(III) displays very little to no toxicity in animals up to the highest dose tested. In the<br />
Shara et al. (2007) study, the only effects observed in rats dietary exposed to 25 mg/kg <strong>chromium</strong><br />
nicotinate were statistically significant decreases in body weight gain, between 5.5 and 14.9 %, which<br />
were not considered as adverse by the CONTAM Panel. The lack of toxicity observed at high<br />
concentrations of Cr(III) may reflect the poor absorption of Cr(III) by the oral route of exposure. The<br />
use of baked bread as a vehicle may have further reduced the absorption of <strong>chromium</strong> in the Ivankovic<br />
and Preussman (1975) study.<br />
The studies conducted by the National Toxicology Program (NTP, 2010) have been performed<br />
according to present scientific guidances and reported a comprehensive assessment of toxicological<br />
endpoints and adequate reporting for estimation of doses in mg Cr(III)/kg b.w. per day. They were<br />
considered by the CONTAM Panel to be the most relevant studies to establish reference doses. No<br />
adverse effects have been observed in the sub-chronic or long-term toxicity studies in mice or rats at<br />
the highest dose tested of 50000 mg CrO 3 or <strong>chromium</strong> picolinate/kg diet. The relevant NOAELs<br />
corresponded to 506 and 286 mg Cr(III)/kg b.w. per day for the sub-chronic and long-term toxicity in<br />
the rat, respectively (NTP, 2010).<br />
EFSA Journal 2014;12(3):3595 71