efsa-opinion-chromium-food-drinking-water
efsa-opinion-chromium-food-drinking-water efsa-opinion-chromium-food-drinking-water
Chromium in food and bottled water Table H3: Summary of in vivo genotoxicity of Cr(III) - oral route Test system/ Endpoint Rat (F344/N) Micronuclei Mouse (B6C3F1) Micronuclei Mouse (BDF1) Micronuclei Rats (Sprague– Dawley) Micronuclei Mouse (C57BL/6J) DNA deletions (pun reversion assay) Compound Cr picolinate Cr picolinate monohydrate Chromic potassium sulphate dodecahydrate CrK(SO 4 ) 2 x 12H 2 O Cr picolinate Cr(III) chloride salt Dose/route Oral exposure by gavage 156 to 2500 mg/kg b.w. Doses: 19.4- 310.7 mg Cr(III))/kg b.w. per day Oral exposure in feed 80 to 50.000 mg/kg diet Doses: M:2-1419 mg Cr(III)/kg b.w. per day F: 1.7-1090 mg Cr(III)/kg b.w. per day Drinking water 500mg/l Doses: M:165 mg Cr(III)/kg b.w. per day F: 140 mg Cr(III)/kg b.w. per day Single oral dose of 33, 250, 2000 mg/kg b.w. Doses: 4.1, 30.8, 246 mg Cr(III)/kg b.w. per day Drinking water to dams 1875 or 3750 mg/l Doses: 375 or 750 mg Cr(III)/kg b.w. day Exposure time/evaluation time three times at 24 hr intervals 3 months feeding for 7 months 18 and 42 hrs after administration Transplacental effect in the embryos harvested at 17.5 days postcoitum Tissue Response* Reference Bone marrow erythrocytes Peripheral blood erythrocytes Bone marrow and peripheral blood cells Bone marrow cells Developing embryos (embryo Cr(III) concentrations were 8.72 and 18.77 ng/g, respectively) Negative 2500 mg Crpic/kg b.w. 310.7 mg Cr(III))/kg b.w. per day Negative - 50.000 mg/kg 1419 mg Cr(III)/kg b.w. per day Negative 165 mg Cr(III)//kg b.w. per day Negative 246 mg Cr(III)//kg b.w. per day Positive 375 mg Cr(III)//kg b.w. per day b.w.: body weight. * The lowest effective dose is indicated for positive results and the highest dose tested for negative results. NTP (2010) NTP (2010) De Flora et al. (2006) Komorowski et al. (2008) Kirpnick- Sobol et al. (2006) EFSA Journal 2014;12(3):3595 192
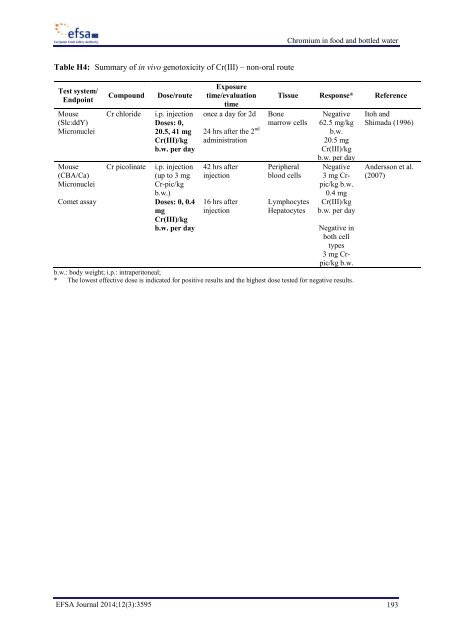
Chromium in food and bottled water Table H4: Summary of in vivo genotoxicity of Cr(III) – non-oral route Test system/ Endpoint Mouse (Slc:ddY) Micronuclei Mouse (CBA/Ca) Micronuclei Comet assay Compound Cr chloride Cr picolinate Dose/route i.p. injection Doses: 0, 20.5, 41 mg Cr(III)/kg b.w. per day i.p. injection (up to 3 mg Cr-pic/kg b.w.) Doses: 0, 0.4 mg Cr(III)/kg b.w. per day Exposure time/evaluation time once a day for 2d 24 hrs after the 2 nd administration 42 hrs after injection 16 hrs after injection Tissue Response* Reference Bone marrow cells Peripheral blood cells Lymphocytes Hepatocytes Negative 62.5 mg/kg b.w. 20.5 mg Cr(III)/kg b.w. per day Negative 3 mg Crpic/kg b.w. 0.4 mg Cr(III)/kg b.w. per day Negative in both cell types 3 mg Crpic/kg b.w. b.w.: body weight; i.p.: intraperitoneal; * The lowest effective dose is indicated for positive results and the highest dose tested for negative results. Itoh and Shimada (1996) Andersson et al. (2007) EFSA Journal 2014;12(3):3595 193
- Page 141 and 142: Chromium in food and drinking water
- Page 143 and 144: Chromium in food and drinking water
- Page 145 and 146: Chromium in food and drinking water
- Page 147 and 148: Chromium in food and drinking water
- Page 149 and 150: Chromium in food and drinking water
- Page 151 and 152: Chromium in food and drinking water
- Page 153 and 154: Chromium in food and drinking water
- Page 155 and 156: Chromium in food and drinking water
- Page 157 and 158: Chromium in food and drinking water
- Page 159 and 160: Chromium in food and drinking water
- Page 161 and 162: Chromium in food and drinking water
- Page 163 and 164: Chromium in food and drinking water
- Page 165 and 166: Chromium in food and drinking water
- Page 167 and 168: Chromium in food and drinking water
- Page 169 and 170: Chromium in food and drinking water
- Page 171 and 172: Chromium in food and drinking water
- Page 173 and 174: Chromium in food and bottled water
- Page 175 and 176: Chromium in food and bottled water
- Page 177 and 178: Chromium in food and bottled water
- Page 179 and 180: Chromium in food and bottled water
- Page 181 and 182: Chromium in food and bottled water
- Page 183 and 184: Chromium in food and bottled water
- Page 185 and 186: Chromium in food and bottled water
- Page 187 and 188: Chromium in food and bottled water
- Page 189 and 190: Chromium in food and bottled water
- Page 191: Chromium in food and bottled water
- Page 195 and 196: Chromium in food and bottled water
- Page 197 and 198: Chromium in food and bottled water
- Page 199 and 200: Chromium in food and bottled water
- Page 201 and 202: Chromium in food and bottled water
- Page 203 and 204: Chromium in food and bottled water
- Page 205 and 206: Chromium in food and bottled water
- Page 207 and 208: Chromium in food and bottled water
- Page 209 and 210: Chromium in food and bottled water
- Page 211 and 212: Chromium in food and bottled water
- Page 213 and 214: Chromium in food and bottled water
- Page 215 and 216: Chromium in food and bottled water
- Page 217 and 218: Chromium in food and bottled water
- Page 219 and 220: Chromium in food and bottled water
- Page 221 and 222: Chromium in food and bottled water
- Page 223 and 224: Chromium in food and bottled water
- Page 225 and 226: Chromium in food and bottled water
- Page 227 and 228: Chromium in food and bottled water
- Page 229 and 230: Chromium in food and bottled water
- Page 231 and 232: Chromium in food and bottled water
- Page 233 and 234: Chromium in food and bottled water
- Page 235 and 236: J.1.2. mice Chromium in food and bo
- Page 237 and 238: Chromium in food and bottled water
- Page 239 and 240: Chromium in food and bottled water
- Page 241 and 242: Chromium in food and bottled water
Chromium in <strong>food</strong> and bottled <strong>water</strong><br />
Table H4: Summary of in vivo genotoxicity of Cr(III) – non-oral route<br />
Test system/<br />
Endpoint<br />
Mouse<br />
(Slc:ddY)<br />
Micronuclei<br />
Mouse<br />
(CBA/Ca)<br />
Micronuclei<br />
Comet assay<br />
Compound<br />
Cr chloride<br />
Cr picolinate<br />
Dose/route<br />
i.p. injection<br />
Doses: 0,<br />
20.5, 41 mg<br />
Cr(III)/kg<br />
b.w. per day<br />
i.p. injection<br />
(up to 3 mg<br />
Cr-pic/kg<br />
b.w.)<br />
Doses: 0, 0.4<br />
mg<br />
Cr(III)/kg<br />
b.w. per day<br />
Exposure<br />
time/evaluation<br />
time<br />
once a day for 2d<br />
24 hrs after the 2 nd<br />
administration<br />
42 hrs after<br />
injection<br />
16 hrs after<br />
injection<br />
Tissue Response* Reference<br />
Bone<br />
marrow cells<br />
Peripheral<br />
blood cells<br />
Lymphocytes<br />
Hepatocytes<br />
Negative<br />
62.5 mg/kg<br />
b.w.<br />
20.5 mg<br />
Cr(III)/kg<br />
b.w. per day<br />
Negative<br />
3 mg Crpic/kg<br />
b.w.<br />
0.4 mg<br />
Cr(III)/kg<br />
b.w. per day<br />
Negative in<br />
both cell<br />
types<br />
3 mg Crpic/kg<br />
b.w.<br />
b.w.: body weight; i.p.: intraperitoneal;<br />
* The lowest effective dose is indicated for positive results and the highest dose tested for negative results.<br />
Itoh and<br />
Shimada (1996)<br />
Andersson et al.<br />
(2007)<br />
EFSA Journal 2014;12(3):3595 193