Ocular rosacea Crotoxin for paralysis of extraocular muscles ...
Ocular rosacea Crotoxin for paralysis of extraocular muscles ... Ocular rosacea Crotoxin for paralysis of extraocular muscles ...
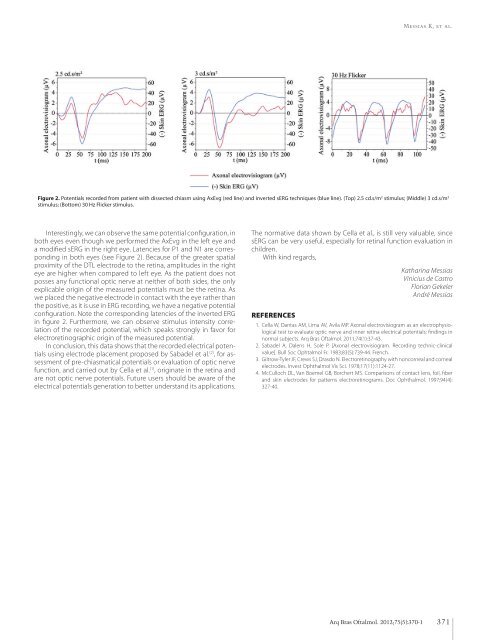
Messias K, et al. Figure 2. Potentials recorded from patient with dissected chiasm using AxEvg (red line) and inverted sERG techniques (blue line). (Top) 2.5 cd.s/m 2 stimulus; (Middle) 3 cd.s/m 2 stimulus; (Bottom) 30 Hz Flicker stimulus. Interestingly, we can observe the same potential configuration, in both eyes even though we performed the AxEvg in the left eye and a modified sERG in the right eye. Latencies for P1 and N1 are corresponding in both eyes (see Figure 2). Because of the greater spatial proximity of the DTL electrode to the retina, amplitudes in the right eye are higher when compared to left eye. As the patient does not posses any functional optic nerve at neither of both sides, the only explicable origin of the measured potentials must be the retina. As we placed the negative electrode in contact with the eye rather than the positive, as it is use in ERG recording, we have a negative potential configuration. Note the corresponding latencies of the inverted ERG in figure 2. Furthermore, we can observe stimulus intensity correlation of the recorded potential, which speaks strongly in favor for electroretinographic origin of the measured potential. In conclusion, this data shows that the recorded electrical potentials using electrode placement proposed by Sabadel et al. (2) , for assessment of pre-chiasmatical potentials or evaluation of optic nerve function, and carried out by Cella et al. (1) , originate in the retina and are not optic nerve potentials. Future users should be aware of the electrical potentials generation to better understand its applications. The normative data shown by Cella et al., is still very valuable, since sERG can be very useful, especially for retinal function evaluation in children. With kind regards, Katharina Messias Vinicius de Castro Florian Gekeler André Messias References 1. Cella W, Dantas AM, Lima AV, Avila MP. Axonal electrovisiogram as an electrophysiological test to evaluate optic nerve and inner retina electrical potentials: findings in normal subjects. Arq Bras Oftalmol. 2011;74(1):37-43. 2. Sabadel A, Dalens H, Sole P. [Axonal electrovisiogram. Recording technic-clinical value]. Bull Soc Ophtalmol Fr. 1983;83(5):739-44. French. 3. Giltrow-Tyler JF, Crews SJ, Drasdo N. Electroretinography with noncorneal and corneal electrodes. Invest Ophthalmol Vis Sci. 1978;17(11):1124-27. 4. McCulloch DL, Van Boemel GB, Borchert MS. Comparisons of contact lens, foil, fiber and skin electrodes for patterns electroretinograms. Doc Ophthalmol. 1997;94(4): 327-40. Arq Bras Oftalmol. 2012;75(5):370-1 371
- Page 28 and 29: Toscano DA, et al. in 1.28 seconds.
- Page 30 and 31: Toscano DA, et al. observed excelle
- Page 32 and 33: Viani GA, et al. METHODS Types of s
- Page 34 and 35: Viani GA, et al. Table 1. Character
- Page 36 and 37: Viani GA, et al. OR: odds ratio. Fi
- Page 38 and 39: Viani GA, et al. domized study whic
- Page 40 and 41: Artigo Original | Original Article
- Page 42 and 43: Magri MPF, et al. Tabela 3. Causas
- Page 44 and 45: Artigo Original | Original Article
- Page 46 and 47: Rodrigues ACL, et al. dias - 96 mes
- Page 48 and 49: Artigo Original | Original Article
- Page 50 and 51: Yaacov-Peña F, et al. recorded if
- Page 52 and 53: Guerra RLL, et al. METHODS A retros
- Page 54 and 55: Guerra RLL, et al. terial endophtha
- Page 56 and 57: Tartarella MB, et al. inactive tumo
- Page 58 and 59: Tartarella MB, et al. 15. Portellos
- Page 60 and 61: Nasser LS, et al. De acordo com a c
- Page 62 and 63: Nasser LS, et al. A heterocromia de
- Page 64 and 65: Magalhães OA, et al. Figure 1. Gel
- Page 66 and 67: Kamei RW O ultrassom mostrava um v
- Page 68 and 69: Relato de Caso | Case Report Buphth
- Page 70 and 71: Artigo de Revisão | Review Article
- Page 72 and 73: Vieira ACC, et al. is that Demodex
- Page 74 and 75: Vieira ACC, et al. is often misdiag
- Page 76 and 77: Vieira ACC, et al. 51. Lazaridou E,
- Page 80 and 81: Instruções para Autores | Instruc
- Page 82 and 83: Capítulos de livros Gómez de Lia
- Page 84 and 85: AGORA A LINHA 1-DAY ACUVUE® MOIST
Messias K, et al.<br />
Figure 2. Potentials recorded from patient with dissected chiasm using AxEvg (red line) and inverted sERG techniques (blue line). (Top) 2.5 cd.s/m 2 stimulus; (Middle) 3 cd.s/m 2<br />
stimulus; (Bottom) 30 Hz Flicker stimulus.<br />
Interestingly, we can observe the same potential configuration, in<br />
both eyes even though we per<strong>for</strong>med the AxEvg in the left eye and<br />
a modified sERG in the right eye. Latencies <strong>for</strong> P1 and N1 are corresponding<br />
in both eyes (see Figure 2). Because <strong>of</strong> the greater spatial<br />
proximity <strong>of</strong> the DTL electrode to the retina, amplitudes in the right<br />
eye are higher when compared to left eye. As the patient does not<br />
posses any functional optic nerve at neither <strong>of</strong> both sides, the only<br />
explicable origin <strong>of</strong> the measured potentials must be the retina. As<br />
we placed the negative electrode in contact with the eye rather than<br />
the positive, as it is use in ERG recording, we have a negative potential<br />
configuration. Note the corresponding latencies <strong>of</strong> the inverted ERG<br />
in figure 2. Furthermore, we can observe stimulus intensity correlation<br />
<strong>of</strong> the recorded potential, which speaks strongly in favor <strong>for</strong><br />
electroretinographic origin <strong>of</strong> the measured potential.<br />
In conclusion, this data shows that the recorded electrical potentials<br />
using electrode placement proposed by Sabadel et al. (2) , <strong>for</strong> assessment<br />
<strong>of</strong> pre-chiasmatical potentials or evaluation <strong>of</strong> optic nerve<br />
function, and carried out by Cella et al. (1) , originate in the retina and<br />
are not optic nerve potentials. Future users should be aware <strong>of</strong> the<br />
electrical potentials generation to better understand its applications.<br />
The normative data shown by Cella et al., is still very valuable, since<br />
sERG can be very useful, especially <strong>for</strong> retinal function evaluation in<br />
children.<br />
With kind regards,<br />
Katharina Messias<br />
Vinicius de Castro<br />
Florian Gekeler<br />
André Messias<br />
References<br />
1. Cella W, Dantas AM, Lima AV, Avila MP. Axonal electrovisiogram as an electrophysiological<br />
test to evaluate optic nerve and inner retina electrical potentials: findings in<br />
normal subjects. Arq Bras Oftalmol. 2011;74(1):37-43.<br />
2. Sabadel A, Dalens H, Sole P. [Axonal electrovisiogram. Recording technic-clinical<br />
value]. Bull Soc Ophtalmol Fr. 1983;83(5):739-44. French.<br />
3. Giltrow-Tyler JF, Crews SJ, Drasdo N. Electroretinography with noncorneal and corneal<br />
electrodes. Invest Ophthalmol Vis Sci. 1978;17(11):1124-27.<br />
4. McCulloch DL, Van Boemel GB, Borchert MS. Comparisons <strong>of</strong> contact lens, foil, fiber<br />
and skin electrodes <strong>for</strong> patterns electroretinograms. Doc Ophthalmol. 1997;94(4):<br />
327-40.<br />
Arq Bras Oftalmol. 2012;75(5):370-1<br />
371