Films minces à base de Si nanostructuré pour des cellules ...
Films minces à base de Si nanostructuré pour des cellules ...
Films minces à base de Si nanostructuré pour des cellules ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
tel-00916300, version 1 - 10 Dec 2013<br />
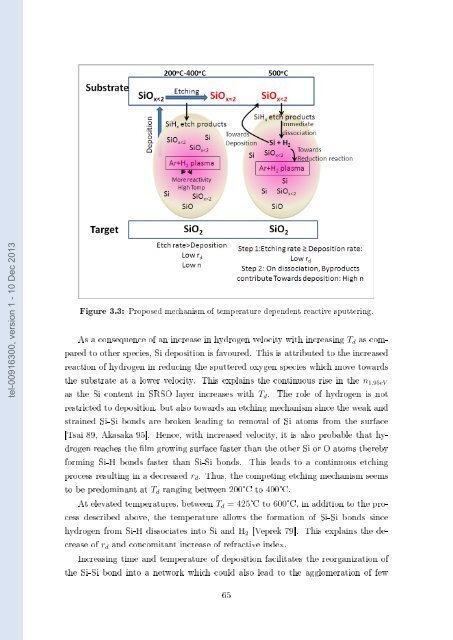
Figure 3.3: Proposed mechanism of temperature <strong>de</strong>pen<strong>de</strong>nt reactive sputtering.<br />
As a consequence of an increase in hydrogen velocity with increasing T d as compared<br />
to other species, <strong>Si</strong> <strong>de</strong>position is favoured. This is attributed to the increased<br />
reaction of hydrogen in reducing the sputtered oxygen species which move towards<br />
the substrate at a lower velocity. This explains the continuous rise in the n 1.95eV<br />
as the <strong>Si</strong> content in SRSO layer increases with T d . The role of hydrogen is not<br />
restricted to <strong>de</strong>position, but also towards an etching mechanism since the weak and<br />
strained <strong>Si</strong>-<strong>Si</strong> bonds are broken leading to removal of <strong>Si</strong> atoms from the surface<br />
[Tsai 89, Akasaka 95]. Hence, with increased velocity, it is also probable that hydrogen<br />
reaches the lm growing surface faster than the other <strong>Si</strong> or O atoms thereby<br />
forming <strong>Si</strong>-H bonds faster than <strong>Si</strong>-<strong>Si</strong> bonds. This leads to a continuous etching<br />
process resulting in a <strong>de</strong>creased r d . Thus, the competing etching mechanism seems<br />
to be predominant at T d ranging between 200°C to 400°C.<br />
At elevated temperatures, between T d = 425°C to 600°C, in addition to the process<br />
<strong>de</strong>scribed above, the temperature allows the formation of <strong>Si</strong>-<strong>Si</strong> bonds since<br />
hydrogen from <strong>Si</strong>-H dissociates into <strong>Si</strong> and H 2 [Veprek 79]. This explains the <strong>de</strong>crease<br />
of r d and concomitant increase of refractive in<strong>de</strong>x.<br />
Increasing time and temperature of <strong>de</strong>position facilitates the reorganization of<br />
the <strong>Si</strong>-<strong>Si</strong> bond into a network which could also lead to the agglomeration of few<br />
65