THE PERIODIC TABLE - Small-Scale Chemistry
THE PERIODIC TABLE - Small-Scale Chemistry
THE PERIODIC TABLE - Small-Scale Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
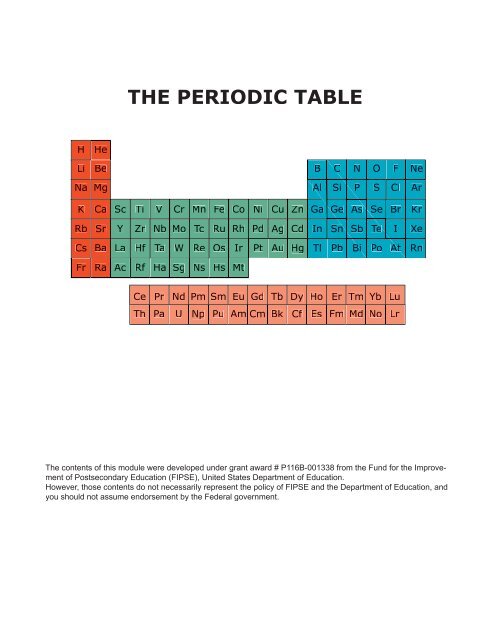
<strong>THE</strong> <strong>PERIODIC</strong> <strong>TABLE</strong><br />
H<br />
He<br />
Li Be B C N O F Ne<br />
Na Mg<br />
Al Si P S Cl Ar<br />
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr<br />
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb<br />
Te I Xe<br />
Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At<br />
Rn<br />
Fr Ra Ac Rf Ha Sg Ns Hs Mt<br />
Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu<br />
Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr<br />
The contents of this module were developed under grant award # P116B-001338 from the Fund for the Improvement<br />
of Postsecondary Education (FIPSE), United States Department of Education.<br />
However, those contents do not necessarily represent the policy of FIPSE and the Department of Education, and<br />
you should not assume endorsement by the Federal government.
<strong>PERIODIC</strong> <strong>TABLE</strong><br />
ROUND <strong>TABLE</strong>S<br />
Groups and Periods<br />
Atomic Radii<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
Group 1A<br />
Group 2A<br />
Group 3A<br />
Group 4A<br />
Group 5A<br />
Group 6A<br />
Group 7A<br />
Group 8A<br />
Transition Metals<br />
Lanthanides and Actinides<br />
0 nm 260 nm<br />
Melting Points<br />
Boiling Points<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
0 K 3300 K 0 K 6000 K
<strong>PERIODIC</strong> <strong>TABLE</strong><br />
ROUND <strong>TABLE</strong>S<br />
Electron Shell Filling<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
New electron in s shell.<br />
New electron in p shell.<br />
New electron in d shell.<br />
New electron in f shell.<br />
Anomalous.<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
This is how the new electrons would fi ll if there were<br />
no irregularities.
Actinium<br />
Ac<br />
Aluminun<br />
Al<br />
Americium<br />
Am<br />
Antimony<br />
Sb<br />
Argon<br />
A<br />
Arsenic<br />
As<br />
Astatine<br />
At<br />
Barium<br />
Ba<br />
Berkelium<br />
Bk<br />
Beryllium<br />
Be<br />
Bismuth<br />
Bi<br />
Bohrium<br />
Bh<br />
Boron<br />
B<br />
Bromine<br />
Br<br />
Cadmium<br />
Cd<br />
Calcium<br />
Ca<br />
Californium<br />
Cf<br />
Carbon<br />
C<br />
Cerium<br />
Ce<br />
Cesium<br />
Cs<br />
Chlorine<br />
Cl<br />
Chromium<br />
Cr<br />
Cobalt<br />
Co<br />
Copper<br />
Cu<br />
Curium<br />
Cm<br />
Dubnium<br />
Db<br />
Dysprosium<br />
Dy<br />
Einsteinium<br />
Es<br />
Erbium<br />
Er<br />
Europium<br />
Eu<br />
Fermium<br />
Fm<br />
Fluorine<br />
F<br />
Francium<br />
Fr<br />
Gadolinium<br />
Gd<br />
Gallium<br />
Ga<br />
Germanium<br />
Ge<br />
Gold<br />
Au<br />
Hafnium<br />
Hs<br />
Hassium<br />
Hs<br />
Helium<br />
He<br />
Holmium<br />
Ho<br />
Hydrogen<br />
H<br />
Indium<br />
In<br />
Iodine<br />
I<br />
Iridium<br />
Ir<br />
Iron<br />
Fe<br />
Krypton<br />
Kr<br />
Lanthanum<br />
La<br />
Lawrencium<br />
Lr<br />
Lead<br />
Pb<br />
Lithium<br />
Li<br />
Lutetium<br />
Lu<br />
Magnesium<br />
Mg<br />
Manganese<br />
Mn<br />
Meitnerium<br />
Mt<br />
Mendelevium<br />
Md<br />
Mercury<br />
Hg<br />
Molybdenum<br />
Mo<br />
Neodymium<br />
Nd<br />
Neon<br />
Ne<br />
Neptunium<br />
Np<br />
Nickel<br />
Ni<br />
Niobium<br />
Nb<br />
Nitrogen<br />
N<br />
Nobelium<br />
No<br />
Osmium<br />
Os<br />
Oxygen<br />
O<br />
Palladium<br />
Pd<br />
Phosphorus<br />
P<br />
Platinum<br />
Pt<br />
Plutonium<br />
Pu<br />
Polonium<br />
Po<br />
Potassium<br />
K<br />
Praseodymium Pr<br />
Promethium<br />
Pm<br />
Protactinium<br />
Pa<br />
Radium<br />
Ra<br />
Radon<br />
Rn<br />
Rhenium<br />
Re<br />
Rhodium<br />
Rh<br />
Rubidium<br />
Rb<br />
Ruthenium<br />
Ru<br />
Rutherfordium<br />
Rf<br />
Samarium<br />
Sm<br />
Scandium<br />
Sc<br />
Seaborgium<br />
Sg<br />
Selenium<br />
Se<br />
Silicon<br />
Si<br />
Silver<br />
Silver<br />
Silver<br />
Ag<br />
Sodium<br />
Na<br />
Strontium<br />
Sr<br />
Sulfur<br />
Sulfur<br />
Sulfur<br />
S<br />
Tantalum<br />
Ta<br />
Technetium<br />
Tc<br />
Tellurium<br />
Te<br />
Terbium<br />
Tb<br />
Thallium<br />
Tl<br />
Thorium<br />
Th<br />
Thulium<br />
Tm<br />
Tin<br />
Sn<br />
Titanium<br />
Ti<br />
Tungsten<br />
W<br />
Uranium<br />
U<br />
Vanadium<br />
V<br />
Xenon<br />
Xe<br />
Ytterbium<br />
Yb<br />
Yttrium<br />
Y<br />
Zinc<br />
Zn<br />
Zirconium<br />
Zr<br />
<strong>PERIODIC</strong> <strong>TABLE</strong><br />
Copy this page. Then, using the copy write in as<br />
many element symbols as you can. Repeat as often<br />
as you wish.<br />
PRACTICE <strong>TABLE</strong>
<strong>PERIODIC</strong> <strong>TABLE</strong><br />
QUANTUM NUMBERS OF MOST RECENTLY ADDED ELECTRON<br />
R<br />
+1⁄2<br />
m s<br />
-1⁄2<br />
m s<br />
= +1⁄2<br />
m s<br />
= -1⁄2<br />
1<br />
0 0<br />
He<br />
2<br />
m s<br />
= +1⁄2<br />
m s<br />
= -1⁄2<br />
+1 0 -1 +1 0 -1<br />
3<br />
4<br />
5<br />
6<br />
7<br />
+2 +1 0 -1 -2 +2 +1 0 -1 -2<br />
*<br />
*<br />
* * * * *<br />
* *<br />
o<br />
6<br />
7<br />
+3 +2 +1 0 -1 -2 -3 +3 +2 +1 0 -1 -2 -3<br />
*<br />
*<br />
* * * * *<br />
s-block<br />
l<br />
= 0<br />
n = R<br />
m s<br />
= +1⁄2<br />
m s<br />
= -1⁄2<br />
p-block<br />
l<br />
= 1<br />
n = R<br />
d-block<br />
f-block<br />
l = 2 n = R-1<br />
l = 3 n = R-2<br />
The magnetic quantum number, m, is the<br />
number from +3 to -3 marked on the top of each<br />
column.<br />
The four quantum numbers of the most recently added<br />
electron can be read from this table; that is, except for<br />
the exceptions, which are marked with an asterisk.<br />
Conversly, given a set of four quantum numbers you can<br />
locate the position of the element on the table.<br />
For example, if<br />
n = 3<br />
L = 1<br />
m = 0<br />
m s<br />
= -1⁄2<br />
then we can fi nd the element where we have placed the<br />
small red ‘o’, which is bromine.<br />
Find the elements which have the following sets of<br />
quantum numbers.<br />
n = 2, L = 0, m = 0, m s<br />
= +1⁄2<br />
n = 4, L = 2, m = +2, m s<br />
= -1⁄2<br />
What are the quantum numbers of:<br />
neon<br />
sodium<br />
vanadium<br />
gallium<br />
hydrogen<br />
oxygen?