Business Overview 2007 - Synthes
Business Overview 2007 - Synthes
Business Overview 2007 - Synthes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Our Values & Principles<br />
Annual Report <strong>2007</strong>
Our Values & Principles<br />
Annual Report <strong>2007</strong>
Preface<br />
Our Values & Principles<br />
This year’s Annual Report focuses upon our Values & Principles.<br />
They capture the inherent culture of <strong>Synthes</strong> and have been,<br />
are, and will be at the core of our activities as a company and<br />
as individuals. Guided by these Values & Principles, we make<br />
every effort to deliver on our mission of improving patient care.<br />
Patient Driven<br />
Surgeon Focus<br />
Striving every day to<br />
improve patient care<br />
Listening to surgeons<br />
and helping them<br />
achieve the best possible<br />
patient outcomes<br />
Innovation<br />
Quality<br />
Constantly developing<br />
breakthrough products,<br />
services, and better<br />
ways of doing business<br />
Maintaining the highest<br />
standard of excellence<br />
in all that we deliver to<br />
internal and external<br />
customers<br />
Education<br />
Partnership<br />
Integrity<br />
Continually educating<br />
and developing<br />
our customers and<br />
employees<br />
Leveraging the insights<br />
of surgeons, the AO,<br />
strategic partners and<br />
our global network<br />
of <strong>Synthes</strong> employees<br />
Acting ethically and<br />
respectfully at all times
<strong>Synthes</strong>. Annual Report <strong>2007</strong>
Content<br />
Company Profile 3<br />
Significant Facts 6<br />
Letter to Shareholders 8<br />
<strong>Business</strong> Report<br />
Management Discussion 13<br />
Regional Highlights 15<br />
Corporate Citizenship<br />
Innovation and Education 23<br />
Employee Structure and Challenges 27<br />
Ethical <strong>Business</strong> Conduct 29<br />
Product Highlights<br />
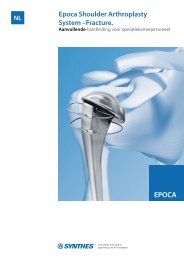
Trauma. LCP Volar Column Plate 32<br />
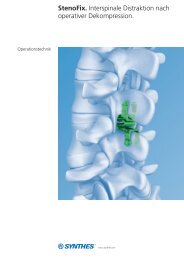
Spine. In-Space 34<br />
CMF. MatrixNEURO Cranial Plating System 36<br />
Biomaterials. chronOS Strip 38<br />
Power Tools. Colibri/Small Battery Drive 40<br />
Corporate Governance<br />
Group Structure and Shareholders 44<br />
Capital Structure 45<br />
Board of Directors 46<br />
Group Management Committee 52<br />
Compensation, Shareholdings and Loans 53<br />
Shareholders’ Participation 55<br />
Changes of Control and Defense Measures 57<br />
Auditors 58<br />
Information Policy 59<br />
1
Company Profile<br />
2
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Company Profile<br />
<strong>Synthes</strong>, headquartered in West Chester, PA (USA), is a leading global medical device company, employing over<br />
9,000 people whose mission is to improve patient care around the world. In <strong>2007</strong>, we generated revenues of<br />
US$ 2.8 billion and net earnings of US$ 612.6 million. Over the last 10 years (1997–<strong>2007</strong>) <strong>Synthes</strong> has increased<br />
its revenues from US$ 364.3 million to US$ 2.8 billion, representing a CAGR of 22.4%, including the acquisition<br />
of Stratec Medical in 1999 and Mathys in 2004.<br />
Through its five business units (Trauma, Spine, Cranio-Maxillofacial,<br />
Biomaterials and Power Tools), <strong>Synthes</strong> develops, produces and markets<br />
instruments, implants and biomaterials for the surgical fixation,<br />
correction and regeneration of the human skeleton and its soft tissues.<br />
We operate in product markets with high growth, driven by the<br />
aging population and improvements in technology that allow treating<br />
more patients with better implants.<br />
<strong>Synthes</strong> establishes the foundations for its excellent market position<br />
by continuously developing better solutions. Our goal is to provide<br />
the safest and most advanced implants, instruments and technologies<br />
that ensure reliable operating procedures, rapid recovery and a<br />
pain-free life after surgery. We guarantee high quality, constant innovation<br />
and total concentration on the needs of our customers.<br />
<strong>Synthes</strong> is the world leader in traumatology, ranks among the top<br />
three companies for spinal devices and is at the forefront of the cranio-maxillofacial<br />
business. We are an innovative pioneer in the field<br />
of biomaterials such as resorbable implants and bone graft substitutes,<br />
and have become a leader in non-fusion technologies with our<br />
lumbar and cervical disc replacements.<br />
Global Functional Structure<br />
North America<br />
Asia Pacific<br />
Europe<br />
Latin America,<br />
Middle East and Africa<br />
Traumatology<br />
Spine<br />
Cranio-Maxillofacial (CMF)<br />
Biomaterials<br />
Power Tools<br />
Production<br />
Production<br />
Global services: Finance, Human Resources, Information Technology, Legal, Purchasing, Quality, Regulatory Affairs<br />
3
Company Profile<br />
Our Strategy<br />
For many years we have been following our three-pillar-strategy,<br />
which is closely related to our Values & Principles:<br />
Innovation<br />
<strong>Synthes</strong> has a constant inflow of new and innovative<br />
products that serve a clinical need<br />
for improved patient care.<br />
Sales Force<br />
The expansion of the number of sales people<br />
and services <strong>Synthes</strong> offers by providing its<br />
sales force with best-in-class training that<br />
they bring into operating rooms around the<br />
globe.<br />
Education<br />
Various activities and educational programs<br />
provided by <strong>Synthes</strong> or by partnering organizations<br />
to foster the best treatment for patients.<br />
<strong>Business</strong> Units<br />
Trauma<br />
As the global market leader in Trauma,<br />
<strong>Synthes</strong> offers plates and screws, intramedullary<br />
nails and external fixators to fix and<br />
stabilize all types of fractures of almost all<br />
bones with the ultimate goal of restoring<br />
normal anatomy and physiology.<br />
Spine<br />
<strong>Synthes</strong> is one of the top companies in<br />
Spine. The primary market is the surgical<br />
management of back pain treatment<br />
which includes fusion procedures with<br />
cages, plates and pedicle screw-systems,<br />
but also non-fusion treatments such as<br />
interspinous spacers, vertebroplasty and<br />
disc arthroplasty.<br />
CMF<br />
<strong>Synthes</strong> has a worldwide leading position<br />
in CMF (Cranio-Maxillofacial). Within this<br />
market, <strong>Synthes</strong> offers very low-profile implants<br />
to treat fractures of the skull and<br />
mandible. The portfolio includes products<br />
to reconstruct mandibular misalignments<br />
and deformities caused by accidents.<br />
Biomaterials<br />
The <strong>Synthes</strong> Biomaterials Division develops solutions that complement<br />
Trauma, Spine and CMF divisions with three particular<br />
areas of focus. Firstly the reduction of implant related infections,<br />
such as drug-coated implants. Secondly, increase the stability of<br />
implants in the bone (such as cements) and thirdly, bone graft<br />
substitutes to promote bony fusion. The products are sold through<br />
the sales forces in Trauma, Spine and CMF.<br />
Power Tools<br />
<strong>Synthes</strong> Power Tools are centrally developed and – like Biomaterials<br />
– used and sold in conjunction with the Trauma, Spine and<br />
CMF divisions. <strong>Synthes</strong> has over 30 years of experience in designing<br />
and manufacturing surgical power tools and offers a comprehensive<br />
range of air- and battery driven drill systems, reamers<br />
and saws to meet high demands in orthopaedics, traumatology<br />
and neurosurgery.<br />
4
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Recent Highlights<br />
December <strong>2007</strong><br />
The US FDA (Food and Drug Administration) fully approves the Pro-<br />
Disc ® -C Total Disc Replacement (a) for commercial sale and distribution<br />
in the United States. <strong>Synthes</strong> is the first company to offer both<br />
a lumbar as well as a cervical artificial disc replacement on the US<br />
market. Thereby <strong>Synthes</strong> strengthens its market position in the segment<br />
of spinal motion preservation. Artificial disc replacements constitute<br />
a modern way of treating degenerative disc disease and will<br />
benefit from a shift in treatment paradigm.<br />
a<br />
December <strong>2007</strong><br />
With the acquisition of N Spine, <strong>Synthes</strong> enters the posterior spinal<br />
dynamic stabilization market to treat degenerative disc disease as well<br />
as stenosis. The N Spine rod will be used in conjunction with <strong>Synthes</strong>'<br />
leading pedicle screw product lines Pangea (b) and Click'X (c). The<br />
benefits are its low profile, which offers a minimal-invasive approach<br />
and ease-of-use.<br />
b<br />
October <strong>2007</strong><br />
In Biomaterials, chronOS Strip (d) is cleared for sale by the FDA. This<br />
is the first flexible ceramic bone substitute <strong>Synthes</strong> has in its portfolio.<br />
The easy-to-use new product offers surgeons increased handling<br />
options and leverages two areas of expertise for <strong>Synthes</strong>: osteoconductive<br />
ceramics and resorbable biocompatible polymers.<br />
c<br />
April <strong>2007</strong><br />
Michel Orsinger is appointed CEO. Prior to joining <strong>Synthes</strong> in 2004<br />
as COO, Mr. Orsinger spent 10 years with Novartis in various executive<br />
management positions, most recently as the CEO and President<br />
of OTC Worldwide. He has a track record of leadership positions and<br />
has been instrumental in transforming <strong>Synthes</strong> into a leading global<br />
company.<br />
d<br />
Financial Highlights <strong>2007</strong> vs. 2006<br />
<strong>2007</strong> 2006<br />
Net Sales US$ 2,759.7 million US$ 2,391.6 million<br />
Gross Profit Margin 81.0% 81.9%<br />
Operating Profit Margin 32.8% 31.9%<br />
Net Earnings Margin 22.2% 21.3%<br />
Free Cashflow US$ 602.0 million US$ 343.6 million<br />
Employees at year-end 9,070 8,451<br />
SWX Swiss Exchange / VirtX<br />
Symbol: SYST.VX<br />
Market Capitalization at year-end <strong>2007</strong>: CHF 16,689 m<br />
5
Company Profile<br />
Significant Facts<br />
Sales per region <strong>2007</strong> (US$ millions)<br />
North America<br />
Europe<br />
Asia Pacific<br />
Rest of world<br />
1,721.0<br />
637.3<br />
248.4<br />
153.0<br />
Asia Pacific 9.0%<br />
Rest of World 5.5%<br />
Europe 23.1% North America 62.4%<br />
Operating Expenses (US$ millions)<br />
Selling and Promotion<br />
% of Net Sales<br />
General and Administrative<br />
% of Net Sales<br />
Research and Development *<br />
% of Net Sales<br />
2003 2004 2005 2006 <strong>2007</strong><br />
331.7 491.7 592.6 704.6 802.3<br />
27.0% 27.6% 28.5% 29.5% 29.1%<br />
113.5 191.2 247.8 266.4 294.6<br />
9.2% 10.7% 11.9% 11.1% 10.7%<br />
281.2 109.5 114.8 125.6 148.6<br />
22.9% 6.2% 5.5% 5.3% 5.4%<br />
* 2003 includes US$ 215.0 million IPR&D write-off due to<br />
acquisition of Spine Solutions, Inc. in April 2003.<br />
US$ millions<br />
900<br />
800<br />
700<br />
600<br />
500<br />
400<br />
300<br />
200<br />
100<br />
0<br />
2003 2004 2005 2006 <strong>2007</strong><br />
Selling and<br />
Promotion<br />
General and<br />
Administrative<br />
Research and<br />
Development<br />
Profitability (US$ millions)<br />
Net Sales<br />
Gross Profit Margin<br />
Operating Profit Margin<br />
Net Earnings Margin<br />
2003 2004 2005 2006 <strong>2007</strong><br />
1,229.0 1,779.3 2,078.2 2,391.6 2,759.7<br />
81.1% 78.2% 82.5% 81.9% 81.0%<br />
18.6% 28.4% 31.9% 31.9% 32.8%<br />
6.1% 18.2% 21.0% 21.3% 22.2%<br />
US$ millions<br />
3,000<br />
2,500<br />
2,000<br />
1,500<br />
1,000<br />
500<br />
0<br />
2003 2004 2005 2006 <strong>2007</strong><br />
Net<br />
Sales<br />
Gross<br />
Profit<br />
Operating<br />
Profit<br />
Net<br />
Earnings<br />
6
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
FCF and Capital Expenditure (US$ millions)<br />
Free Cashflow<br />
CapEx<br />
2003 2004 2005 2006 <strong>2007</strong><br />
203.3 301.1 337.1 343.6 602.0<br />
122.3 141.3 184.0 190.3 223.1<br />
US$ millions<br />
700<br />
600<br />
500<br />
400<br />
300<br />
200<br />
100<br />
0<br />
2003 2004 2005 2006 <strong>2007</strong><br />
Free<br />
Cashflow<br />
CapEx<br />
Assets <strong>2007</strong> (US$ millions)<br />
Current Assets<br />
Property, Plant & Equipment<br />
Intangible Assets<br />
Goodwill<br />
Other Assets<br />
1,588.4<br />
603.4<br />
1,843.1<br />
1,051.3<br />
101.8<br />
Goodwill 20.3%<br />
Other Assets 2.0%<br />
Current Assets 30.6%<br />
Intangible Assets 35.5%<br />
Property, Plant &<br />
Equipment 11.6%<br />
Liabilities and Equity <strong>2007</strong> (US$ millions)<br />
Current Liabilities<br />
608.7<br />
Long-term Liabilities<br />
493.3<br />
Retained Earnings<br />
1,834.7<br />
Other Equity<br />
2,251.3<br />
Other Equity 43.4%<br />
Current Liabilities 11.7%<br />
Long-term Liabilities 9.5%<br />
Retained Earnings 35.4%<br />
Employees 2003 2004 2005 2006 <strong>2007</strong><br />
Total Employees (at year-end)<br />
4,290 6,711 7,627 8,451 9,070<br />
Change in Employees<br />
13% 56% 14% 11% 7%<br />
Sales Force as % of Total Employees<br />
24% 25% 25% 25% 27%<br />
10,000<br />
8,000<br />
6,000<br />
4,000<br />
2,000<br />
0<br />
2003 2004 2005 2006 <strong>2007</strong><br />
60%<br />
50%<br />
40%<br />
30%<br />
20%<br />
10%<br />
0%<br />
Total<br />
Employees<br />
Sales<br />
Force<br />
Change in<br />
Employees %<br />
7
Letter to Shareholders<br />
Letter to Shareholders<br />
<strong>2007</strong> was another successful year for <strong>Synthes</strong>. We enhanced our leading market position in both Trauma and<br />
Cranio-Maxillofacial specialties. We also acquired N Spine to further strengthen our position in Spine. On an<br />
organizational level, we created new global functions to increase productivity and to gain efficiency as a strategic<br />
response to the changing market environment.<br />
Dear shareholders, employees and friends of <strong>Synthes</strong><br />
We look back with satisfaction on a successful <strong>2007</strong>. It is with great<br />
pleasure that we report net sales of US$ 2.8 billion, representing an<br />
increase of 15.4% versus 2006. All business units contributed to the<br />
success of the <strong>2007</strong> results. The higher-than-market sales growth of<br />
Trauma and CMF both represent a particularly satisfactory achievement<br />
given the large worldwide market share <strong>Synthes</strong> has in these<br />
segments.<br />
Within our Trauma division, we expanded our global product portfolio<br />
in <strong>2007</strong> with the introductions of the Volar Column Distal Radius<br />
Plate System, the Expert Lateral Entry Femoral Recon Nail, the Japanese<br />
Proximal Femoral Nail and further penetration of our LCP systems.<br />
We also implemented several customer-focused programs that<br />
have contributed to our successful year. The first of these programs<br />
is the <strong>Synthes</strong> Resident Program, an online educational platform for<br />
young surgeons in the field of Trauma. <strong>Synthes</strong> also established several<br />
Geriatric Fracture Centers of Excellence in the US this past year<br />
and will continue to expand the program in 2008 worldwide. The<br />
Geriatric program aims to improve the treatment of elderly fracture<br />
patients by developing innovative products for complex fractures,<br />
educating the orthopedic community on the benefits of treating patients<br />
using an interdisciplinary approach, and ultimately establishing<br />
centers which utilize co-managed care and standardized protocols.<br />
Notable activities within Spine in <strong>2007</strong> include the US Food and Drug<br />
Administration’s approval for sale of ProDisc-C and our acquisition of<br />
N Spine. With the approval of ProDisc-C, our product for cervical total<br />
disc replacement, we became the only Spine Company to offer<br />
both lumbar and cervical artificial disc replacement systems. In spite<br />
of the slower than expected initial adoption of this revolutionary technology,<br />
we are confident that artificial disc replacements will prevail<br />
as the preferred treatment option. Our acquisition of N Spine has enhanced<br />
our position within the dynamic stabilization segment of the<br />
lumbar Spinal market by offering an innovative posterior system compatible<br />
with our Click’X and Pangea pedicle screw product lines. We<br />
also look forward to further developing spine technologies with the<br />
addition of the N Spine team to the <strong>Synthes</strong> organization.<br />
Our Cranio-Maxillofacial business outpaced the aggregate global<br />
market through stronger than average growth in each region. Of particular<br />
note was the performance of the neurosurgery segment,<br />
driven by the new MatrixNEURO cranial plating system, and global<br />
success with Patient Specific Implants. In <strong>2007</strong>, the CMF business<br />
group also expanded the content and frequency of specialized technical<br />
educational activities in the Sternal/Chest Wall and Neurotrauma<br />
fields, further establishing <strong>Synthes</strong> as a partner in patient care<br />
worldwide.<br />
8
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Strategic initiatives<br />
<strong>Synthes</strong> continues to make progress with our key global initiatives to<br />
reduce the time-to-market of new product launches, to further optimize<br />
the global supply chain, and to ensure a global product lifecycle<br />
management tool to more efficiently develop and launch new products<br />
across the globe. Our efforts with these key initiatives are critical<br />
to positively position ourselves within the challenges of our everchanging<br />
market environment of shorter product lifecycles and higher<br />
regulatory hurdles for product approvals.<br />
At <strong>Synthes</strong>, the highest professional and ethical standards are essential.<br />
In <strong>2007</strong>, the medical technology industry was subject to significant<br />
governmental scrutiny with regards to the relationship between<br />
companies and their surgeon customers and we continuously review<br />
and reinforce our global compliance guidelines with our employees.<br />
Resident Program in North America will be expanded to other divisions<br />
and to Europe. In Spine, we will leverage the acquisition of N Spine,<br />
but also launch important new additions to the product portfolio.<br />
Finally, we will also utilize new technologies in CMF to expand<br />
our offering to maxillofacial and cardiothoracic surgeons, ear-noseand-throat<br />
and plastic surgeons and also neurosurgeons. These portfolios<br />
will be enhanced with complimentary products from our Biomaterials<br />
and Power Tools divisions.<br />
<strong>Synthes</strong>’ success depends on the skills and commitment of our employees<br />
whose ongoing contribution we deeply appreciate. In particular,<br />
we want to thank you, our shareholders and our other stakeholders<br />
for your continued support and confidence in <strong>Synthes</strong>.<br />
In <strong>2007</strong>, the executive management team was further enhanced with<br />
the transition of Michel Orsinger to President and CEO, the addition<br />
of a global Vice President of Operations and a global Vice President<br />
of Human Resources.<br />
Outlook<br />
We have begun 2008 with high confidence for the continuing success<br />
of our company in delivering on our mission of improving patient<br />
care. The majority of our achievements in <strong>2007</strong> were driven by<br />
our commitment to innovation, education and to the high service level<br />
delivered by our own sales force. Going forward, we will maintain<br />
our special focus on innovation with differentiated products and<br />
growth in all regions where we see yet unrealized potential. We remain<br />
optimistic about the future growth opportunities in all our fields<br />
of activities. Furthermore, we expect our global initiatives to enhance<br />
our efficiency and to help sustain our gross and operating margins.<br />
Hansjörg Wyss<br />
Chairman of the Board<br />
Michel Orsinger<br />
President and CEO<br />
In Trauma, <strong>Synthes</strong> will further strengthen its market position by introducing<br />
several new products. The successfully implemented <strong>Synthes</strong><br />
9
<strong>Business</strong> Report<br />
10
<strong>Synthes</strong>. Annual Report 2006<br />
<strong>Business</strong> Report<br />
Management Discussion 13<br />
Regional Highlights 15<br />
11
<strong>Business</strong> Report. Management Discussion<br />
Patient Driven<br />
Striving every day to<br />
improve patient care<br />
12
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
<strong>Business</strong> Report. Management Discussion<br />
<strong>Synthes</strong> once again posted double-digit sales growth of 15.4% in US-Dollars and 12.5% in local currencies.<br />
This performance is a result of our best-in-class products, high service levels and a comprehensive array of educational<br />
activities, which we conducted throughout the world.<br />
North America continued to be the major growth engine for <strong>Synthes</strong>,<br />
with Trauma as the main contributor to this achievement. Outside the<br />
US, performance in Asia Pacific is particularly noteworthy, where in<br />
<strong>2007</strong> we achieved market leadership in the Japanese Trauma market.<br />
China and India have become the fastest growing countries for sales<br />
of <strong>Synthes</strong> products in the emerging Asia markets.<br />
In spite of important investments in research and development spending<br />
as a percentage of sales, we have been able to grow our operating<br />
income by 17.0% in local currencies. Productivity improvement<br />
programs contributed to a reduction in selling and promotion and<br />
general and administrative expenses as a percentage of sales. In addition,<br />
royalty payments to the AO Foundation declined.<br />
In Europe, where competition and pressure on pricing and reimbursement<br />
of our products has increased, we were able to grow our business<br />
significantly with 13.4% local currency growth versus 2006. Latin<br />
America continued with strong sales growth at approximately<br />
19% in local currencies, with particularly strong growth in Spine.<br />
A significant share of our aggregate sales growth (approximately<br />
45%) is attributed to new products providing increased benefits and<br />
products which cover needs previously unmet.<br />
Gross profit margin in <strong>2007</strong> (absent inventory obsolescence expense)<br />
represents a slight improvement over prior year. This is among the<br />
highest in the industry. Our continuous progress in manufacturing efficiency<br />
helped make this development possible despite our expansion<br />
into countries with relatively lower market prices and our inventory<br />
reduction program, which was evident in <strong>2007</strong>.<br />
Net earnings of US$ 612.6 million improved as a percentage of sales<br />
to 22.2% for the year, representing 18.7% growth in local currencies.<br />
Foreign exchange rate differences favorably impacted sales and<br />
earnings by 2.9 and 1.7 percentage points, respectively, in <strong>2007</strong> and<br />
2006. Earnings per share increased by 16.2% in local currencies to<br />
US$ 5.16.<br />
In <strong>2007</strong>, we increased our staffing by 7.3% to 9,070 employees, with<br />
the majority of new hires in our sales organization. Our global sales<br />
force now consists of approximately 2,400 highly educated and trained<br />
sales personnel.<br />
13
<strong>Business</strong> Review. Regional Highlights<br />
Surgeon Focus<br />
Listening to surgeons<br />
and helping them<br />
achieve the best possible<br />
patient outcomes<br />
14
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
<strong>Business</strong> Report. Regional Highlights<br />
In <strong>2007</strong>, <strong>Synthes</strong> achieved considerable growth in all regions. Sales in North America rose to US$ 1.7 billion<br />
(+ US$ 195.9 million) with Trauma and CMF performing particularly well and Spine contributing substantially.<br />
In Europe, <strong>Synthes</strong> increased its sales by 13.4% (in local currencies) to US$ 637.3 million and achieved market<br />
leadership in its three major divisions. Countries in Asia Pacific showed double-digit sales growth rates except<br />
for Japan. In Latin America, sales increased by almost 19%.<br />
North America<br />
In North America we achieved US$ 1.7 billion or 62.4% of our <strong>2007</strong><br />
consolidated net sales. Once again, this region was our biggest<br />
growth contributor with a growth of 12.6% over the prior year in local<br />
currencies. This was mostly due to our Trauma and CMF divisions<br />
where we outgrew the market and gained share despite our marketleading<br />
position.<br />
In Trauma, the Volar Column Distal Radius Plate System and the Expert<br />
Lateral Entry Femoral Recon Nail Systems were two notable systems<br />
released in <strong>2007</strong>. Regional performance was mainly fueled by further<br />
penetration and portfolio expansion of our LCP (Locking Compression<br />
Plate) systems, along with the performance from our Trochanteric<br />
Fixation Nail (TFN) and our Retrograde/Antegrade Femoral Nail<br />
(RAFN) intramedullary nailing systems.<br />
Other important accomplishments within the Trauma division in <strong>2007</strong><br />
included our successful implementation of the <strong>Synthes</strong> Resident Program<br />
in the US. This program is both an online training center and a<br />
series of workshops offered by our sales force directly to young trauma<br />
surgeons who can benefit from education by the market leader<br />
and earn credit points that can later be redeemed for textbooks. By<br />
the end of <strong>2007</strong>, more than 70% of all US Residents were already<br />
enrolled in the <strong>Synthes</strong> Resident Program. This program will be extended<br />
to Spine and CMF in the coming year.<br />
Notable new product launches in our Spine division in <strong>2007</strong> include<br />
Antegra, a new plating system used to stabilize degenerated lumbar<br />
spine segments anteriorly, and Synapse, a comprehensive system of<br />
instruments and implants designed for posterior stabilization of the<br />
upper spine. The continued rollout of SynFix-LR contributed strongly<br />
to our North American Spine growth this past year.<br />
Our acquisition of the N Spine organization in December was a highlight<br />
for our Spine division. N Spine’s unique and proprietary technology<br />
will allow us to enter the dynamic stabilization market, one of<br />
the fastest growing segments of non-fusion technology in Spine.<br />
Since the launch of ProDisc-L in the fall of 2006, there have been<br />
more than 1,000 surgeries in the US in <strong>2007</strong>, which resulted in an<br />
even lower complication rate than during the FDA study. This is a testament<br />
to our training program, and a similar surgeon training strategy<br />
will be applied to ProDisc-C which was approved for sale by the<br />
FDA at the end of <strong>2007</strong>. The major challenge has been and continues<br />
to be the adequate reimbursement by insurance companies, but we<br />
continue to focus on this area and have had success in <strong>2007</strong> with more<br />
insurance companies covering the cost of treatment with ProDisc-L. It<br />
is with confidence that we look into a promising 2008 for Spine.<br />
In our CMF division, the MatrixNEURO system was a strong growth<br />
driver this past year. This system was fully released in the US and will<br />
be expanded to other indications in 2008. It complemented continued<br />
growth with our Patient Specific Implants (PSI). CMF also grew<br />
through increased clinical treatment with its sternal fixation and reconstruction<br />
system.<br />
Another highlight of <strong>2007</strong> was the continued expansion of the <strong>Synthes</strong><br />
Inventory Management Solution (SIMS). By the end of <strong>2007</strong>, over<br />
80% of our US volume is handled through SIMS, which is comprised<br />
of a storage system as well as a computerized solution to make reorders<br />
as easy as possible for the hospital.<br />
In the US we have a very streamlined training concept in place for our<br />
surgeons through our partnership with AO Foundation. We also complement<br />
AO educational activities with specialized courses sponsored<br />
by <strong>Synthes</strong>. A larger number of AO courses was offered in the<br />
North America in <strong>2007</strong>, e.g. the first AO cadaver course in CMF and<br />
other more specialized courses. We will continue to expand on this<br />
fruitful concept and our collaboration with the AO to remain the number<br />
one provider of excellent education and scientific platforms.<br />
Overall, we are dedicated to increasing our high-level of service to<br />
customers by offering total customer solutions in all our activities, including<br />
technical service, inventory management and training.<br />
Europe<br />
Despite an unusually warm winter with fewer fractures associated<br />
with skiing and other accidents typical of snow and icy conditions,<br />
we achieved 13.4% growth in local currencies in Europe. The mild<br />
temperatures affected sales in the traditional winter sport countries<br />
of Germany, Switzerland and Austria. The relatively slow start to the<br />
year was made up for with above average sales performance after<br />
the first quarter.<br />
15
<strong>Business</strong> Review. Regional Highlights<br />
<strong>2007</strong> is the first year in which we achieved market leadership in all<br />
three major divisions, Trauma, Spine and CMF. Continuous expansion<br />
of the sales force and far reaching educational activities have been<br />
one of the largest contributing factors to success in the past year. During<br />
the latter half of <strong>2007</strong> we were able to further strengthen our<br />
product portfolio with new product introductions. The high standard<br />
of our sales force has increased our presence in the operating rooms<br />
and also our ability to train OR staff on the use of our products to<br />
help improve patient outcomes.<br />
One of our goals has been to overcome the changing environments<br />
in our major European markets. Price increases have only been possible<br />
through new product introductions and holding prices of existing<br />
products at previous levels has only been possible through the<br />
strengthening of our value added services. The roll-out of the electronic<br />
<strong>Synthes</strong> Inventory Management Solution (eSIMS) across the major<br />
markets such as the United Kingdom and Germany has further<br />
strengthened our presence at the hospital level. Further, the change<br />
in the reimbursement system in France (TIPS) has given us the opportunity<br />
to launch new products in the local Trauma market.<br />
Once again, we have outperformed market growth in all divisions and<br />
have gained market share throughout Europe. A strong performance<br />
in the United Kingdom, good performance in the Eastern European<br />
markets and the countries on the Mediterranean rim have made up<br />
for the below average first quarter. The completion of the Expert Nail<br />
product lines, the introduction of the LCP DHS and LCP DHS Blade<br />
and the introduction of the EPOCA shoulder prosthesis have driven<br />
growth in the Trauma division.<br />
Our Spine sales have been strengthened through the successful introduction<br />
of In-Space and Vertecem and the line additions to products<br />
launched in 2006, such as Pangea. The introduction of a milling<br />
system has vastly improved the surgical procedure for Prodisc-C.<br />
In <strong>2007</strong>, our CMF division achieved the highest growth percentage.<br />
The introduction of the MatrixNEURO system was the main driver behind<br />
this positive performance while the launch of the Sternal Closure<br />
systems and the improvement in the supply chain of the Patient<br />
Specific Implant (PSI) have also contributed to the strong CMF growth.<br />
The region of Africa and the Middle East has also shown increased<br />
sales and stability. Since the Middle East markets are strongly influenced<br />
by tender activity, much of our sales in <strong>2007</strong> stem from tenders<br />
won in 2006. With the tenders awarded in <strong>2007</strong> for delivery in<br />
2008, it is clear that in this region we have gained significant market<br />
share as well. A re-organization of our distribution arrangements in<br />
large markets, such as Turkey and Greece, has been completed and<br />
will contribute to strong growth in the coming years.<br />
Going forward, we will continue our drive to expand our sales force<br />
and increase our educational opportunities. It is important to support<br />
this team with a strong middle management layer which our Human<br />
Resources group is currently establishing as a high priority. New product<br />
introductions will continue to support our growth in 2008. In<br />
order to get closer to the needs of our surgeons and their patients,<br />
we have established new Innovation Centers in Europe with the most<br />
recent being the Innovation Center in Salzburg, Austria, opened in<br />
October of <strong>2007</strong>. Situated in the Hansjörg Wyss House of the Paracelsus<br />
University, this Center is ideally located to offer surgeons the<br />
platform to work closely with our engineers in order to bring new<br />
products and new surgical solutions to the market.<br />
Asia Pacific<br />
We are pleased with our <strong>2007</strong> results in Asia Pacific. Although our<br />
growth was stymied by ongoing price cuts in Japan which resulted in<br />
a sales growth rate of 8.7% (in local currencies) for the full year, the<br />
rest of the region showed a double-digit sales growth rate as well as<br />
a double digit increase in volume.<br />
Prices in Japan are completely controlled by the Ministry of Health,<br />
Labor and Welfare (MHLW). The MHLW implemented two price cuts<br />
in <strong>2007</strong> (January and April) that affected our Trauma result in Japan<br />
quite significantly (10%–15%). Nevertheless our achievement in Japan<br />
continues to be strong: the market share of <strong>Synthes</strong> was expanded<br />
in all divisions and we are now the market leader in Trauma.<br />
Exceptional growth was achieved in both China and India. The challenge<br />
in these countries lies within the supply of service through the<br />
education of our employees and our distributors, and also in our competition<br />
with locally produced products.<br />
16
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Innovation<br />
Constantly developing<br />
breakthrough products,<br />
services, and better<br />
ways of doing business<br />
17
<strong>Business</strong> Review. Regional Highlights<br />
Quality<br />
Maintaining the highest<br />
standard of excellence<br />
in all that we deliver to<br />
internal and external<br />
customers<br />
18
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
A milestone for the region was the inauguration of the CMF business<br />
in India. This was done in conjunction with an AO course and a <strong>Synthes</strong><br />
symposium on sub-condylar fractures at the International Congress<br />
for Oral and Maxillofacial Surgery in Bangalore, where 800 surgeons<br />
attended. These events gave our CMF division a real jump-start and<br />
we are looking forward to adding people, service and products to<br />
serve our CMF customers in India.<br />
Latin America<br />
Once again our result in Latin America was outstanding. In local currencies,<br />
sales in this region grew by almost 19%. This result was primarily<br />
due to our strong performance in Brazil and Colombia. There<br />
is still great potential in Latin America because of the large population,<br />
continued economic growth and the relative political stability of<br />
the region as a whole.<br />
Another highlight was our launch in the first half of <strong>2007</strong> of the PFNA<br />
Japan (Proximal Femoral Nail Anti-rotational nail) which was a great<br />
success. This is our first product which is specifically adapted for the<br />
unique qualities of the Asian femoral anatomy; it is an intramedullary<br />
nail used to treat proximal fractures of the femur.<br />
The AO has created a dedicated group of surgeons to consider Asiaspecific<br />
training and product needs. With the establishment of the<br />
AO Asia Pacific Trauma Expert Group, we look forward to an expanded<br />
offering of dedicated Asia-specific product lines with the addition<br />
of tibia plates, distal radius plates and an orthognathic CMF system.<br />
In order to more appropriately react to market needs, we have created<br />
two additional sub-regions that are led by separate <strong>Synthes</strong> managers<br />
(North-Asia and South-East Asia). This should allow us to train<br />
the people in our sales force, along with our distributors’ employees<br />
in an equally professional way. We will also improve our response time<br />
by increasing the number of inventory holding offices, as well as accelerating<br />
our rate of new product introduction. New offices with<br />
better warehousing capabilities as well as training facilities have already<br />
been opened in Singapore, India, Malaysia, China and Australia.<br />
Our goal is clearly to expand our activities in this important region.<br />
Apart from the launch of new products and more educational activities,<br />
we will also focus on individual country-specific challenges. One<br />
such challenge is to establish better reimbursement environments in<br />
countries where this is not yet the case (such as India), and also to<br />
provide adequate solutions for an ever increasing number of people<br />
with osteoporosis in the emerging markets. This will be a major task<br />
for us for the coming years.<br />
Six countries in Latin America have <strong>Synthes</strong> sales organizations; nine<br />
other countries are covered by distributorship agreements, and two<br />
distributors service the Caribbean region.<br />
The Spine business unit has shown the most growth as the result of<br />
new product introductions combined with close cooperation with AO<br />
Spine Latin America. Power Tools also showed exceptional growth<br />
due to specialized training of the sales force and the support from a<br />
dedicated regional repair facility based in Costa Rica.<br />
Our results in some countries are influenced by our long-term strategy<br />
of sustainable, ethical business operations. <strong>Synthes</strong> has a clear policy<br />
that does not allow for kickbacks and other unprincipled business<br />
practices and we will always operate according to these principles.<br />
Apart from the increase in our sales force to keep up with the increased<br />
demand, we now have an excellent training organization in place.<br />
It allows for our own trainers to train other local trainers and make<br />
sure that product and technical knowledge is distributed evenly<br />
among our sales people and surgeons.<br />
Our relationship with the AO Foundation continued to be very strong.<br />
With their help almost 2,500 surgeons have been trained in over 50<br />
courses in <strong>2007</strong>. We are also complementing AO courses with <strong>Synthes</strong>-courses<br />
that are more technology and new product oriented.<br />
We expect this region to continue to grow at a substantially higher<br />
rate than more mature markets. In addition, we expect to increase<br />
market share as a result of the training programs mentioned above,<br />
combined with other initiatives that improve the level of service and<br />
overall efficiency of our Latin American operations.<br />
19
Corporate Citizenship<br />
20
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Corporate Citizenship<br />
Innovation and Education 23<br />
Employee Structure and Challenges 27<br />
Ethical <strong>Business</strong> Conduct 29<br />
21
Corporate Citizenship. Innovation and Education<br />
Education<br />
Continually educating<br />
and developing<br />
our customers and<br />
employees<br />
22
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Corporate Citizenship. Innovation and Education<br />
Innovation and education have always been main pillars of <strong>Synthes</strong>' strategy and will always be so in the future.<br />
Innovations by <strong>Synthes</strong> are always based on clinical problems. Our product development centers and<br />
innovation task forces allow us to respond to the increasing demand for innovative solutions. <strong>Synthes</strong>’ ongoing<br />
commitment to education permeates all aspects of our interaction with surgeons and OR personnel. In <strong>2007</strong>,<br />
several of our educational initiatives were launched or further developed.<br />
In <strong>2007</strong>, as in the past, clinical problems were the engine behind<br />
<strong>Synthes</strong>' innovations. Perpetual innovation is reflected in an extensive<br />
pipeline of ideas and in numerous new solutions for clinical problems.<br />
<strong>Synthes</strong> is determined to proactively search for clinical challenges and<br />
tries to solve them, in close cooperation with our clinical and scientific<br />
partners.<br />
Surgical treatment of musculoskeletal diseases<br />
Over the last 50 years, the treatment of musculoskeletal disorders has<br />
evolved and dramatically improved the clinical outcome and patients'<br />
lives. Innovations by <strong>Synthes</strong> are always based on scientific findings,<br />
clinical experience and patient needs. Therefore, our engineers and<br />
project team members understand the clinical needs and are aware<br />
of today’s limits in treating musculoskeletal disorders. To ensure the<br />
involvement of the best leaders in all these disciplines, our engineers<br />
and designers are part of one of the largest worldwide medical and<br />
scientific networks.<br />
The surgical treatment of musculoskeletal diseases is no longer a pioneering<br />
effort, as it has evolved into a well accepted and proven treatment<br />
therapy. This transition was only possible, with the use of standardized<br />
treatment methods and high quality products which work<br />
well in the hands of the majority of trained surgeons.<br />
Today, the needs for innovative solutions have increased. The reason<br />
for this growth, on one side, is the longer lifespan, patient expectation<br />
and the specialization of the surgical professionals. On the other<br />
side, new technologies in the field of materials, biomaterials, diagnostics<br />
and visualization are very promising tools for improving the<br />
surgical treatment.<br />
Development teams located globally<br />
In <strong>2007</strong>, <strong>Synthes</strong> reacted to the increased need for innovation by substantially<br />
extending our development resources, especially in the early<br />
stages of emerging technologies and new treatment methods. To<br />
understand the needs of our local surgical partners, the locations of<br />
our development teams are spread globally. By doing so, we can keep<br />
our focus and dedication as a competent and pro-active partner for<br />
the medical community.<br />
On the one hand, we have dedicated product development centers<br />
for all our divisions, both in the US and in Europe. Engineers and business<br />
people in these organizations make sure that the right innovations<br />
reach the right surgeons at the right time.<br />
On the other hand, to further maintain this awareness and to be even<br />
closer to the real surgical environment, some of <strong>Synthes</strong>' project<br />
teams are physically located at medical centers and at universities.<br />
The resulting interdisciplinary collaboration is fast-paced and very<br />
effective in developing innovative solutions for clinical problems yet<br />
unsolved.<br />
From <strong>Synthes</strong>’ history we have learned that innovations do not end<br />
with the development of a product. Evaluating the clinical results and<br />
proactively exchanging those results with our clinical network are necessary<br />
to be able to validate new implants, instruments, or surgical<br />
techniques. This process ensures that an innovation fulfills the high<br />
standard of a <strong>Synthes</strong> product. For any new and innovative surgical<br />
technique or product, extensive educational programs for surgeons<br />
and OR personnel are key elements in these product releases.<br />
Educational offers for surgeons and OR staff<br />
<strong>Synthes</strong> has always placed a very strong emphasis on educating surgeons<br />
and operating room personnel in a way that compliments the<br />
well-known and highly respected tradition of AO education activities.<br />
During its 50-year history, the AO has developed effective channels<br />
for educating surgeons and OR personnel on AO goals, principles,<br />
and techniques. The AO emphasis on education as a key pillar of its<br />
success made it an early ingredient of the <strong>Synthes</strong> culture and has<br />
now become a major reason for <strong>Synthes</strong>’ success and excellent<br />
reputation.<br />
<strong>Synthes</strong>’ ongoing commitment to education permeates all aspects of<br />
our interaction with surgeons and OR personnel. The most common<br />
form of education that <strong>Synthes</strong> offers can be found on a daily basis<br />
in operating rooms worldwide as highly trained <strong>Synthes</strong> sales consultants<br />
provide valuable technical expertise to the surgeons and OR<br />
staff who use their products. <strong>Synthes</strong> also provides more refined and<br />
more formalized education activities and events, as well as web-based<br />
resources, fellowships, and other opportunities for surgeons to<br />
observe and practice new technologies.<br />
<strong>Synthes</strong> Resident Program to be expanded<br />
Several of <strong>Synthes</strong>’ educational initiatives originated in the United<br />
23
Corporate Citizenship. Innovation and Education<br />
States, and they are now spreading to other areas of the world. An<br />
example is the <strong>Synthes</strong> Resident Program (SRP), a comprehensive educational<br />
program for surgical residents in the United States to enhance<br />
the learning they receive at their teaching institutions.<br />
Introduced in early <strong>2007</strong>, the SRP features technique-specific courses<br />
that allow orthopaedic residents the opportunity to practice and<br />
review their fracture fixation skills. The main element of the program<br />
is a web site that provides on-line courses and resources, including<br />
interactive simulated surgical procedures. The local <strong>Synthes</strong> sales consultant<br />
is actively involved in the SRP, conducting hands-on workshops<br />
in the hospitals with the residents.<br />
Residents who participate in the SRP earn “points” in recognition of<br />
their participation in both the on-line and live learning activities. These<br />
points are used toward the acquisition of medical textbooks that<br />
are a valuable resource that they can use for their entire medical career.<br />
The SRP has been quite well received, with 90% of American<br />
orthopaedic residents utilizing the program. The SRP will be expanded<br />
to <strong>Synthes</strong> Spine and <strong>Synthes</strong> CMF, as well as into the European<br />
and Latin American markets in 2008.<br />
focused on surgical access and less invasive surgical techniques featuring<br />
<strong>Synthes</strong> Spine products.<br />
<strong>Synthes</strong> CMF provides programs consistent with <strong>Synthes</strong>’ commitment<br />
to education. Workshops and courses for neurosurgeons and<br />
cardiothoracic surgeons were conducted in <strong>2007</strong> to educate surgeons<br />
on new <strong>Synthes</strong> products and technologies which are not in the<br />
standard AO product portfolio.<br />
On-line course offerings for OR personnel<br />
Education for operating room personnel (ORP) has been one of <strong>Synthes</strong>’<br />
most well-known attributes, with thousands of nurses, technicians,<br />
and other ORP being educated by local sales consultants and<br />
at workshops and courses worldwide. In <strong>2007</strong>, <strong>Synthes</strong> continued its<br />
tradition of supporting this important part of the surgical team by enhancing<br />
its on-line course offering. These courses enable ORP to learn<br />
and practice fracture fixation techniques and to become familiar<br />
with the instruments and implants using interactive simulation technology.<br />
Participants earn continuing education credit for these online<br />
courses without the need to travel or take time away from their<br />
busy work schedules.<br />
Training in new technologies<br />
Another form of education offered by <strong>Synthes</strong> is the Advanced Technology<br />
Symposia. These Symposia, focused on new technologies that<br />
have not yet been included in the AO Education curriculum, are designed<br />
to give participants hands-on experience with new <strong>Synthes</strong><br />
products. Surgeons who attend this 1-day course, which is conducted<br />
locally in their area, are given the opportunity to be briefed on the<br />
new technology by surgeon experts and to practice the new products<br />
on plastic bones or cadaver specimens. Hundreds of these courses<br />
were conducted world wide in <strong>2007</strong> for all <strong>Synthes</strong> specialties.<br />
Consistent with the Advanced Technology Symposia in <strong>2007</strong> was the<br />
focus on surgeon training for ProDisc-L. Spine surgeons were trained<br />
in small forums allowing for highly individualized and interactive training.<br />
These one-and-a-half day forums featured lecture presentations,<br />
in-depth review of surgical cases (provided by the participants),<br />
hands-on training on cadaver specimens, and observation of a live<br />
ProDisc surgical procedure via interactive video. This intensive ProDisc<br />
training was usually preceded by other <strong>Synthes</strong> surgeon courses that<br />
Finally, <strong>Synthes</strong> participates in numerous professional society meetings<br />
around the world, where we sponsor hands-on learning activities<br />
for the meeting participants. Professional societies appreciate<br />
<strong>Synthes</strong>’ unique commitment to education and they have learned to<br />
expect <strong>Synthes</strong> to deliver the finest quality education for their members.<br />
<strong>2007</strong> was no exception to this trend, as <strong>Synthes</strong> provided education<br />
at society meetings for all major surgeon and ORP specialties<br />
that we serve.<br />
<strong>Synthes</strong>’ commitment to education is without equal in the markets<br />
we serve. In <strong>2007</strong>, <strong>Synthes</strong> conducted over 1,600 courses worldwide,<br />
with over 30,000 participants. This effort, combined with the<br />
highly regarded courses offered by the AO, is a remarkable testimony<br />
to the importance of education within the <strong>Synthes</strong> core values and<br />
principles.<br />
24
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Activities <strong>2007</strong> (1,540)<br />
Latin America 4%<br />
Asia Pacific 5%<br />
North America 13%<br />
Europe 78%<br />
Participants <strong>2007</strong> (34,219)<br />
Asia Pacific 19%<br />
Latin America 5% North America 6%<br />
Europe 70%<br />
25
Corporate Citizenship. Employee Structure and Challenges<br />
Partnership<br />
Leveraging the insights<br />
of surgeons, the AO,<br />
strategic partners and<br />
our global network<br />
of <strong>Synthes</strong> employees<br />
26
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Corporate Citizenship. Employee Structure and Challenges<br />
On December 31, <strong>2007</strong> <strong>Synthes</strong>, Inc. employed more than 9,000 employees worldwide. With targeted programs,<br />
<strong>Synthes</strong> has continued to expand its workforce to support its strategic priorities in the areas of Product<br />
Development and Sales. <strong>Synthes</strong> is focused on attracting, retaining and developing the best talents in the industry<br />
and on fostering the individual potential of all its employees through continuing education, job rotation,<br />
job enrichment and empowerment.<br />
During <strong>2007</strong> <strong>Synthes</strong> grew its global headcount by more than 7%.<br />
As of December 31, <strong>2007</strong>, <strong>Synthes</strong> employed 9,070 staff in about 40<br />
countries around the world. Globally, the alignment to our strategy<br />
of sales consultant expansion and the delivery of superior technical<br />
support and service led to a 15% increase in our Field Sales organization.<br />
The continuing focus on operational efficiencies such as the<br />
introduction of Lean Manufacturing and Value Stream Mapping allowed<br />
our global Operations group to support the volume resulting<br />
from our sales growth with minimal additional headcount. Our ongoing<br />
expansion in the strategic growth markets of the Asia Pacific<br />
and Latin America regions resulted in double-digit growth in employees<br />
in those locations.<br />
Structure of <strong>Synthes</strong>’ global workforce in %<br />
Other professionals 21%<br />
Manufacturing/<br />
Operations 43%<br />
Product Development 9%<br />
Sales 27%<br />
In the prospect of further global expansion our aim is to offer attractive<br />
opportunities for ambitious and highly skilled individuals who want<br />
to realize their potential in the fields of applied science, engineering,<br />
technical operations, manufacturing, sales and business management.<br />
To maintain a leading position, we must continue to attract,<br />
retain and develop the best talents in the industry. It is of utmost importance<br />
that our talent pool reflects the diverse structure of our business<br />
both now and for the future.<br />
Our global New Employee Orientation program is designed for the<br />
quick integration of new employees, providing them with an understanding<br />
of the company’s heritage, global course, and the values<br />
and principles we will maintain in reaching our goals. This ensures<br />
that new employees quickly understand what contribution they make<br />
to the development of our products and services.<br />
Enhancing global understanding<br />
To support the global development of <strong>Synthes</strong>, we have, and will continue,<br />
to increase our investment in the number of employees active<br />
in our international assignment program. As part of our ongoing efforts<br />
to build and share knowledge, drive innovation and refine our<br />
global business processes, the program supports employees on both<br />
short and long-term assignments to <strong>Synthes</strong> operations around the<br />
world. During <strong>2007</strong> the number of employees on assignment grew<br />
by more than 25%.<br />
At our many sites around the world, we actively strive to maintain an<br />
exciting and modern working environment that makes internal<br />
growth and development a priority. Our human resources policy is<br />
centered around fostering the individual potential of employees<br />
through continuing education and development programs to help<br />
them reconcile career and private goals. Our ambitious efforts in these<br />
areas make us an attractive employer worldwide.<br />
As an organization, <strong>Synthes</strong> understands that our success is the result<br />
of highly talented, motivated and trained individuals coming together<br />
with a common purpose. We maintain our commitment to<br />
their growth, well-being and success.<br />
Attracting and retaining talents<br />
It remains a priority at <strong>Synthes</strong> to attract young talent to the organization.<br />
We are expanding our partnerships with some of the world’s<br />
leading business schools to bring recent graduates to the company.<br />
Through a structured MBA recruitment and development initiative,<br />
individuals experience a 24-month rotational development program<br />
that equips them with experience and insights across major disciplines<br />
within the company.<br />
27
Corporate Citizenship. Ethical <strong>Business</strong> Conduct<br />
Integrity<br />
Acting ethically and<br />
respectfully at all times<br />
28
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Corporate Citizenship. Ethical <strong>Business</strong> Conduct<br />
Ethical behavior is the cornerstone of how <strong>Synthes</strong> does business. Our Global Code of <strong>Business</strong> Conduct and<br />
Ethics guides our, daily decisions and interactions that define how we treat and do business with our customers,<br />
shareholders, suppliers, stakeholders, partners, and each other. The corresponding Compliance program<br />
ensures that business practices and decisions are in line with our ethical values.<br />
At <strong>Synthes</strong> we value the trust and respect of our patients, customers,<br />
and of the medical community. We believe that our reputation is our<br />
greatest asset; an important and integral part of our <strong>Synthes</strong> brand.<br />
To maintain our reputation, we are dedicated to fairness, dignity and<br />
respect, to open and honest relationships with our customers, affiliated<br />
physicians and business partners, to the responsible and efficient<br />
use of our assets. These are keys to our mission as a business, and<br />
are central responsibilities for our employees.<br />
A guide to <strong>Synthes</strong>’ global standards of ethics and<br />
regulatory compliance<br />
Striving to operate with the utmost integrity has long been integral<br />
to our success. Our Global Code of <strong>Business</strong> Conduct and Ethics provides<br />
us with a single, company-wide statement and with an ethical<br />
and behavioral framework to guide our actions. Organized into six<br />
broad principles, our Global Code guides our actions across all of our<br />
activities. It elaborates on aspects such as the responsibility of the individual<br />
employee and the relationship among our employees; the relationships<br />
with our customers, vendors, suppliers and other contractors;<br />
as well as with regulators, communities and competitors.<br />
Whenever we encounter ethical issues, our employees are required<br />
to take personal responsibility to respond in a manner that reflects<br />
the shared values promoted by the Global Code of <strong>Business</strong> Conduct<br />
and Ethics. Through their contracts with us, we require our distributors<br />
and similarly-situated business partners to join us in these principles<br />
as well.<br />
From guidelines to action<br />
To assure ethical or legal compliance and to support our Global Code,<br />
we have developed our corporate compliance program, a system of<br />
standards and procedures, education and training, communication<br />
efforts, monitoring techniques, employee incentives and policy enforcement<br />
designed to facilitate consistent, company-wide adherence<br />
to our Global Code and other standards.<br />
In order to obtain the legal benefits of good corporate citizenship,<br />
we have also designed our corporate compliance program to satisfy<br />
defined government and industry standards for effective compliance<br />
programs around the world. These standards include the United<br />
States Government’s Sentencing Commission Guidelines, applicable<br />
provisions include the Swiss Code of Obligations, the Australian Standard<br />
- 3806-1998 (Compliance Programs), the Organization for Economic<br />
Cooperation and Development’s Guidelines for Multinational<br />
Enterprises, and the International Organization for Standardization’s<br />
standards for quality management systems. With all the above initiatives,<br />
we feel that <strong>Synthes</strong> has reaffirmed and strengthened its commitment<br />
to strong Ethical <strong>Business</strong> Conduct practices throughout the<br />
organization.<br />
To achieve its goals, our program is organized<br />
around seven basic functions:<br />
1. We have assigned oversight responsibility to our Chief Compliance<br />
Officer, and have provided him with the authority, independence<br />
and resources to implement an effective compliance function.<br />
Our Chief Compliance Officer reports directly to the Chairman of<br />
our Board of Directors and to the Audit Committee of our Board.<br />
2. We have established and continue to develop compliance standards<br />
and procedures that are designed to effectively prevent, detect<br />
and address instances of ethical and legal non-compliance.<br />
These standards include appropriate standards and procedures relevant<br />
to each country in which we operate our business.<br />
3. In conjunction with our Legal, Human Resources and Training Departments,<br />
we design and regularly conduct employee training<br />
programs.<br />
4. We regularly encourage communication between our employees<br />
and those persons who can best answer their questions or concerns.<br />
5. We monitor the compliance of business operations, and audit specific<br />
areas of potential compliance risk, to ensure adherence to<br />
compliance-related standards and procedures.<br />
6. We promote and enforce our standards and procedures through<br />
providing employees with incentives to comply, and by applying<br />
consistent and effective discipline when non-compliance occurs.<br />
7. We review potential instances of non-compliance promptly and<br />
appropriately, and responding appropriately. All investigations are<br />
performed by or under the supervision of our Chief Compliance<br />
Officer or our Legal Department.<br />
29
Product Highlights<br />
30
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Product Highlights<br />
Trauma. LCP Volar Column Plate 32<br />
Spine. In-Space 34<br />
CMF. MatrixNEURO Cranial Plating System 36<br />
Biomaterials. chronOS Strip 38<br />
Power Tools. Colibri/Small Battery Drive 40<br />
31
Product Highlights. Trauma<br />
Trauma. LCP Volar Column Plate<br />
The Locking Compression Plate (LCP) Distal Radius System offers 28 individual locking compression plates to manage<br />
every wrist injury pattern that requires surgical intervention. It consists of dorsal and volar plates for fractures<br />
and osteotomies of the wrist, i.e. the distal radius. The LCP Volar Column Distal Radius Plate, a recent addition to<br />
the successful product line, provides extra advantages which strengthen our leading position with this versatile product<br />
line.<br />
Today’s trauma surgeon is faced with an increasing number of complex<br />
injuries. The situation is all the more challenging given that patients<br />
have higher expectations of making a quick and complete recovery.<br />
Further, an aging population means poor bone quality in many<br />
cases and a steady increase in fracture incidences.<br />
Fractures of the wrist, i.e. the distal radius, occur more frequently<br />
than any other single fracture in humans. A fall on an outstretched<br />
hand is the most common mechanism of injury and its incidence is<br />
increasing as people grow older and stay active longer. While nondisplaced<br />
fractures can still be treated conservatively, displaced fractures<br />
usually require active intervention.<br />
Locking plates as new standard<br />
Historically, that intervention has included a combination of closedreduction<br />
and casting, percutaneous pinning and casting, external fixation,<br />
and non-locking plate and screw fixation. Treatment of the<br />
more complex fracture patterns was usually managed with either external<br />
fixation or non-locking plates and screws.<br />
With the advent of locked plating, open reduction and internal fixation<br />
with locking plates and screws were shown to have short-term<br />
advantages over external fixation in these complex fractures. Postoperative<br />
care was less complicated when using internal locking plates<br />
and screws. These advantages have resulted in this treatment method<br />
becoming the new standard of care for distal radius fractures.<br />
Anatomically contoured volar distal radius plates<br />
<strong>Synthes</strong> released the LCP Distal Radius System to the market in early<br />
2004. The LCP Volar Column Distal Radius Plate was released in <strong>2007</strong><br />
to compliment the existing product line. The product offering includes<br />
a total of twelve new Volar Column plates that provide the surgeon<br />
with many options to treat a wide variety of distal radius fracture<br />
patterns.<br />
Three Column Theory<br />
Since Rikli and Regazzoni have shown the majority of the axial load<br />
transmitted through the wrist is concentrated on the intermediate<br />
column, the new Volar Column Distal Radius Plate was designed to<br />
provide more distal buttressing capability and focus sufficient locking<br />
screw options into the intermediate column. At the same time, additional<br />
locking screw fixation options are also directed into the radial<br />
styloid. The remainder of the locking screw holes are evenly distributed<br />
across the distal radius to ensure that there are sufficient locking<br />
screw options to address almost any fracture pattern.<br />
The LCP combination holes in the shaft of the plate allow for either<br />
compression or angular stability. Surgeons can decide intraoperatively<br />
on the best strategy for treating the fracture. In the case of simple<br />
fractures, the system permits compression with standard screws<br />
when primary healing is desired. In more complex fractures with significant<br />
bone defects or in patients with poor bone quality, locking<br />
screws can be locked into the plate to provide stable fracture fixation<br />
independent of bone quality. Locking Screws increase stability, minimize<br />
the risk of the screws stripping in bone, and allow for secondary<br />
healing. The increased stability permits immediate mobilization<br />
and allows the patient to begin early rehabilitation.<br />
With the addition of the LCP Volar Column Plate, <strong>Synthes</strong>’ LCP Distal<br />
Radius System strengthens its position in the market as the most<br />
comprehensive system for treating distal radius fractures. It helps surgeons<br />
to substantially improve surgical outcomes for their patients.<br />
In the near future, <strong>Synthes</strong> will introduce several new instruments and<br />
an upgraded case assortment to compliment the Volar Column Plate<br />
and minimize time spent in the operating room.<br />
Each plate is designed and manufactured to provide an anatomical<br />
fit on the volar aspect of the distal radius, which minimizes the need<br />
for intra-operative plate contouring. The screw trajectories of the Volar<br />
Column plates were developed to focus locking screw fixation options<br />
where they are needed most.<br />
32
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
Radial Column<br />
Intermediate Column<br />
Ulnar Column<br />
Recent studies show that more axial load is transmitted<br />
through the intermediate column of the distal radius.<br />
Therefore, the Volar Column Distal Radius Plate was designed<br />
to provide more buttressing capability and focus<br />
sufficient locking screw options into the intermediate<br />
column.<br />
33
Product Highlights. Spine<br />
Spine. In-Space<br />
Lumbar Spinal Stenosis (LSS) usually affects middle-aged and older adults with debilitating pain in their buttocks or<br />
legs. The traditional surgical procedure to relieve patients from pain caused by lumbar spinal stenosis involves removing<br />
the bone and soft tissues of the spine that are pinching the nerves. In-Space is an implant that facilitates<br />
the interspinous distraction by widening the openings that accommodate the nerves or the spinal cord.<br />
LSS is a widespread pathology which leads to decreased physical activity<br />
levels. The patients most often experience numbness, weakness,<br />
or cramping while standing upright or walking for any distance. This<br />
leg pain is intensified when the patient bends backwards and decreases<br />
when the patient sits down or leans forward. The reason for this<br />
is a compression of neural structures in the posterior part of the spine<br />
when it is in extension. It is caused by a tendency of the foramen<br />
(openings where the nerve roots exit the spine or canal that accommodates<br />
the spinal cord) to narrow in the elderly population. At some<br />
stage, these openings cannot accommodate the nerve roots or spinal<br />
cord any longer without causing pain. Bending backwards (extending)<br />
narrows these openings even more, which explains the intensifying<br />
of pain in this situation.<br />
People in their 60s or 70s are typical patients suffering from Lumbar<br />
Spinal Stenosis (LSS). In fact, LSS is the most common indication for<br />
surgery in people over 60 years old in the United States. It is estimated<br />
that as many as 250,000 to 500,000 people in the US alone have<br />
symptoms of spinal stenosis. This represents about 5 of every 1000<br />
Americans older than 50 years and this number is expected to grow<br />
as members of the baby boomer generation reach their 60s over the<br />
next decade. The trend is global, with the majority of the world’s older<br />
persons residing in Asia (53 percent), and Europe having the next<br />
largest share (25 percent).<br />
spinous processes in a procedure that generally does not last longer<br />
than twenty minutes. It acts as a mechanical block that prevents the<br />
painful extension of the spine and slightly distracts the affected spinal<br />
segment on the back, thus widening the openings that accommodate<br />
the nerve roots or the spinal cord. This is achieved with much<br />
less damage to muscles and ligaments, since the device is implanted<br />
percutaneously through a tiny incision on the side and through a small<br />
tube, leaving all the important structures intact. The implant is secured<br />
to prevent migration through wings, as shown on the images.<br />
These wings are deployed using the insertion instrument, after the<br />
implant has reached its correct position. Since the implant additionally<br />
decreases the pressure on the posterior part of the disc and the<br />
facet joints, it can help patients with leg pain as well as patients with<br />
pain in the lower back.<br />
The following case study (courtesy of Prof. Dr. M. Mayer, Munich)<br />
illustrates the working principle of In-Space.<br />
Surgical treatment of LSS<br />
These patients can oftentimes be helped by an intervention called<br />
surgical decompression. This procedure involves removing the bone<br />
and soft tissues of the spine that are pinching the nerves in order to<br />
make those openings wider again to relieve pressure on the nerve<br />
roots. The disadvantage of this intervention is the need for an open<br />
surgery with all the risks involved. Further, many important structures<br />
surrounding the spine (muscles, ligaments) are compromised during<br />
the decompression surgery. Depending on the extent of the decompression,<br />
the stability of the treated spine segment itself may also<br />
be compromised.<br />
Recent developments include implants used in surgery to treat the<br />
symptoms of spinal stenosis, while preserving as much normal motion<br />
in the spine as possible. In-Space, a new spine implant from <strong>Synthes</strong>,<br />
offers a superior surgical solution. In-Space is implanted between the<br />
Case study This 53-year old male patient had low back pain. He<br />
rated the pain at 5 on the VAS 10-point scale (Visual Analogue Pain<br />
Intensity Scale) and 39 points on the Oswestry Disability Index (ODI)<br />
for 6 months. Diagnosed with spinal intermittent claudication, this<br />
patient had a walking range of 500 metres. Conservative treatment,<br />
including epidural injections was unsuccessful. In July 2006, the<br />
patient underwent percutaneous implantation of an In-Space type of<br />
interspinous spacer and reported pain relief immediately after<br />
surgery. One year later, the patient rated the pain at VAS
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
a<br />
b<br />
c<br />
d<br />
Fig. a–d: Lumbar spine X-rays:<br />
pre-operative/postoperative comparison: noticeable<br />
correction of retro-listhesis. Correction of hyperlordosis.<br />
Enlarged posterior disc height and intervertebral<br />
foramen.<br />
35
Product Highlights. Cranio-Maxillofacial<br />
Cranio-Maxillofacial. MatrixNEURO Cranial Plating System<br />
The <strong>Synthes</strong> MatrixNEURO system contains a complete line of implants (plates and screws) and instruments. The<br />
plating system is intended for a wide variety of cranial indications such as craniotomies, cranial trauma repair and<br />
reconstruction. The system offers several advantages for both the surgeon and the patient in comparison to traditional<br />
surgical methods.<br />
The MatrixNEURO system is intended for the treatment of a wide variety<br />
of cranial indications, e.g. craniotomy, cranioplasty, skull base<br />
surgery, depressed skull fracture. A craniotomy is the most common<br />
open procedure performed by neurosurgeons to achieve access to<br />
the brain. It is defined as the surgical removal of a section of bone<br />
(bone flap) from the skull for the purpose of operating on the underlying<br />
tissues. Over 1,000,000 craniotomies are performed each year<br />
around the world to relieve pressure inside the skull and to treat neurologic<br />
diseases and disorders such as tumors, aneurysms, vascular<br />
malformations, seizures, and strokes.<br />
First, the screws self-retain to the screwdriver blade and are self-drilling<br />
for quick insertion and fast closure of bone flaps. The plates and<br />
mesh are available in a wide variety of shapes and sizes which offer<br />
manifold options for surgeons to treat numerous indications. The plates<br />
and screws are low in profile which provides better cosmetic results<br />
and minimal palpation in areas of the head with very little soft<br />
tissue coverage. The MatrixNEURO system also includes ergonomic<br />
instrumentation for the neurosurgeon which is housed in a modular<br />
case, providing the hospital flexibility in the storage of neuro fixation<br />
instruments and implants.<br />
Craniotomies are good examples of a procedure where the use of the<br />
MatrixNEURO is beneficial. Once the neurosurgical repair has been<br />
performed, the bony anatomy must be restored to its original position.<br />
The traditional method of fixating the cranial bone flap includes<br />
drilling a series of holes around the bone flap and the patient’s native<br />
skull. Stainless steel wire or suture material is then passed through<br />
the holes to secure the bone flap in place. Although this technique<br />
is well-established and initially provides acceptable closure of the<br />
bone flap, it has many disadvantages. Wire closure is tedious and<br />
time-consuming and may be insufficient to provide long-term stability,<br />
especially in cases of severe trauma or bone infection.<br />
Patients having cranial procedures desire to return to their normal<br />
lives without obvious physical signs of their surgical experiences. The<br />
low profile <strong>Synthes</strong> MatrixNEURO cranial plating system not only provides<br />
excellent long-term stable fixation but also an aesthetically pleasing<br />
cosmetic result.<br />
Advanced cranial fixation procedures<br />
The use of plates and screws – a standard method of bone repair in<br />
orthopaedics for more than 100 years – recently has been incorporated<br />
into cranial fixation procedures. The MatrixNEURO system offers<br />
a complete line of plates, screws and mesh designed specifically<br />
for neurosurgical fixation. This system offers many advantages over<br />
traditional methods.<br />
36
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
MatrixNEURO is a cranial plating system which offers<br />
a variety of plates, screws, and instruments intended for<br />
the treatment of cranial indications. It provides longterm<br />
stable fixation and an aesthetically pleasing cosmetic<br />
result.<br />
37
Product Highlights. Biomaterials<br />
Biomaterials. chronOS Strip<br />
Bone void fillers are scaffold materials that fill traumatically or surgically created gaps in the skeletal system. They<br />
help prevent soft tissue in-growth during the healing process while simultaneously providing a pathway through<br />
which new bone can form. chronOS Strip is an innovative ceramic composite offering surgeons improved handling<br />
options over existing bone void fillers during spine fusions. It leverages two areas of expertise for <strong>Synthes</strong>: osteoconductive<br />
ceramics and resorbable biocompatible polymers.<br />
A common practice in spine fusions has been for surgeons to transplant<br />
bone from a patient’s own iliac crest (hip) to the area of the spine<br />
that is to be fused. This adjunct procedure to the spine fusion surgery<br />
is referred to as autografting. While it offers the surgeon more<br />
bone material to place at the fusion site, autografting usually requires<br />
a second surgical incision site and often leaves the patient with<br />
long-term pain in the hip from where the autograft bone was removed.<br />
As an alternative to autografting, synthetic bone void fillers were developed.<br />
They provide a medium for new bone growth while eliminating<br />
the need for the second surgical site and subsequent longterm<br />
hip pain. Beta tricalcium phosphate based ceramics perform well<br />
in this role since they are composed of the same basic elements found<br />
in natural bone, are biocompatible, and provide a uniform, threedimensional<br />
pore structure that can be perfused with bone marrow<br />
aspirated from the patient’s vertebral body or from the iliac crest.<br />
With chronOS Strip, <strong>Synthes</strong> offers surgeons an osteoconductive ceramic<br />
bone void filler that is 100% synthetic, easy to manipulate to<br />
precisely fill the intended skeletal void, and an ideal carrier for a patient’s<br />
own bone marrow. Due to the unique combination of materials,<br />
chronOS Strip contains no human or animal derived substances.<br />
Further, the implant can be rolled, folded, cut or sutured intraoperatively,<br />
and then conformed to each patient’s anatomy. Finally, an innovative<br />
perfusion pack facilitates the process of saturating the implant<br />
with a patient’s own bone marrow or blood. The perfusion pack<br />
can be connected to any standard syringe, such as the one found in<br />
the <strong>Synthes</strong> Bone Marrow Aspiration System.<br />
The unique features of chronOS Strip give surgeons the ability to offer<br />
patients a safe and effective alternative to autografting procedures.<br />
Need for an osteoconductive and flexible ceramic implant<br />
A major drawback of standard ceramics however, is that they are by<br />
nature rigid and brittle and are therefore difficult to precisely shape<br />
to fit the skeletal structure. Intraoperatively, this means that a surgeon<br />
must either start with a larger ceramic preform and meticulously<br />
cut and burr it to fit the bone void, or pack small granules of the ceramic<br />
into the void until it is adequately filled. Neither process is ideal.<br />
The smaller granules can be difficult to handle and keep localized in<br />
the bone void while the shaping of a larger preformed ceramic is often<br />
tedious and time-consuming.<br />
To address this clinical need, <strong>Synthes</strong> has designed a ceramic implant<br />
that provides significant handling advantages over existing ceramic<br />
preforms and granules. chronOS Strip is osteoconductive, i.e. it provides<br />
a pathway through which new bone can form, and is replaced<br />
with new bone in 6 to 18 months during the bone remodeling process.<br />
Being a composite of <strong>Synthes</strong> chronOS and a resorbable, biocompatible<br />
polymer, it leverages two areas of expertise for <strong>Synthes</strong>:<br />
chronOS (beta tricalcium phosphate) is already effectively used to treat<br />
trauma, spine and maxillofacial indications, while neuro, plastic,<br />
and maxillofacial surgeons have relied on resorbable screws, plates,<br />
and tacks from <strong>Synthes</strong> Cranio-Maxillofacial for many years.<br />
38
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
chronOS Strip has several handling advantages over<br />
existing ceramic bone void fillers traditionally utilized<br />
in spine fusion surgeries. It is easily shaped to precisely<br />
fill the skeletal void and stays localized once placed.<br />
39
Product Highlights. Power Tools<br />
Power Tools. Colibri/Small Battery Drive<br />
The universal battery Power Tool system called Colibri or Small Battery Drive has been one of the star products in<br />
the Power Tools portfolio to date. Suitable for a large range of applications, it is light, ergonomic and versatile. Several<br />
new system components have been added over the years to provide surgeons with additional advantages.<br />
Thanks to its unique features, this tool system has become the standard in many countries.<br />
Colibri stands for Compact Light Battery Drill. "Small Battery Drive"<br />
(the name used for this universal battery Power Tool system in the<br />
US) highlights that this product is light and handy, and driven by a<br />
small battery. Besides the excellent ergonomics and easy handling<br />
there are particular features that make this power tool unique.<br />
First, <strong>Synthes</strong> batteries do not have a memory effect. Our high-tech<br />
charging technology eliminates the undesirable memory effect common<br />
with Nickel Cadmium and (to a lesser extent) also Nickel Metal<br />
Hydride batteries. Therefore, the <strong>Synthes</strong> batteries have a long usage<br />
time during the surgery, even after several months of use.<br />
Reliable protection of soft tissue<br />
With its well-balanced hand piece, the Colibri /Small Battery Drive is<br />
optimally designed for the anatomy of the hand, and soft tissue is<br />
protected thanks to the drill’s integrated oscillating mode. In this<br />
mode, the drill bit is not rotating but only oscillating around its own<br />
axis. This feature is highly beneficial for patients; because the drill bit<br />
never makes a whole turn, soft tissues and nerves cannot get wrapped<br />
around the drill bit. In comparison to other available systems, the<br />
hand piece of the Colibri/Small Battery Drive is very stable. Even when<br />
the oscillating mode is used, it provides absolute stability in the surgeon’s<br />
hand.<br />
Versatile use<br />
The system contains an extensive range of attachments to cover a variety<br />
of uses. Trauma, foot, hand, paediatric, spine, veterinary and<br />
cranio-maxillofacial surgeons all benefit from the wide range of attachments<br />
which are designed to fit perfectly with the various <strong>Synthes</strong><br />
implant systems, thus providing optimal performance in practice.<br />
The patented Oscillating Saw Attachment II was launched in 2006<br />
and offers a new surgical technique for treating hallux valgus deformities<br />
(deformation of the big toe). The major advantage of this oscillating<br />
saw attachment is its cannulation which allows the setting<br />
of a guide wire to create a half moon shaped cut, i.e. the crescentic<br />
osteotomy for correcting the hallux valgus. The ability to check the<br />
orientation of the cut to be made lowers the risk of a depression or<br />
elevation of the toe after the surgery and makes the crescentic technique<br />
feasable for the average surgeon.<br />
Second, <strong>Synthes</strong> batteries are not sterilised, but inserted into a sterile<br />
battery case before the surgery, eliminating the need for reprocessing<br />
and sterilization of the battery, and allowing quick reuse with<br />
full capacity. This procedure and our high-tech charging technology<br />
guarantee the longest life time of batteries available on the market.<br />
As a result, we offer a guarantee of one full year to our customers.<br />
Extension of attachment range<br />
In 2008, the attractiveness of the Colibri/Small Battery Drive system<br />
will be further enhanced by the introduction of additional attachments.<br />
New attachments with a higher drilling speed and new chucks<br />
with a higher reaming torque are expected to be launched in mid<br />
2008. An additional new feature will be the color coding of attachments<br />
which will improve the visual differentiation in the operating<br />
room.<br />
In the veterinary field, the Oscillating Saw Attachment III for the “tibia<br />
plateau leveling osteotomy” widely used in canine surgery will also<br />
be launched in mid-year 2008. This saw attachment, together with<br />
the corresponding new crescentic saw blades, will make this technique<br />
much easier and will offer for the first time the possibility for<br />
veterinarian surgeons to use one power system for all cutting procedures<br />
during this type of surgery.<br />
With these further extensions and improvements of its unique features,<br />
the Colibri / Small Battery Drive system will provide additional advantages<br />
for surgeons and further enhance its popularity worldwide.<br />
Durable and powerful batteries<br />
Long-lasting and powerful batteries in two different sizes ensure that<br />
optimal power is provided according to the surgeon’s needs. Depending<br />
on the application – simple or more demanding surgeries –<br />
powerful or less powerful batteries can be employed. Surgeons benefit<br />
from two advantages:<br />
40
<strong>Synthes</strong>. Annual Report <strong>2007</strong><br />
The Colibri/Small Battery Drive system is unparalleled<br />
on the market and enjoys great popularity among surgeons<br />
globally. A wide attachment range offers a variety<br />
of uses in many different types of surgery.<br />
41
This annual report is published in English and in<br />
German. The original language is English.<br />
Concept and design<br />
<strong>Synthes</strong>, Marketing Services & Communications<br />
Oberdorf, Switzerland<br />
<strong>Synthes</strong>, Investor Relations<br />
Solothurn, Switzerland<br />
Ute Drewes, Visuelle Gestaltung und Illustration<br />
Basel, Switzerland<br />
Photography<br />
Fotoarchiv Robert Bösch<br />
Oberägeri, Switzerland<br />
Lithography<br />
<strong>Synthes</strong>, Marketing Services & Communications<br />
Oberdorf, Switzerland<br />
Desktop publishing<br />
Ute Drewes, Visuelle Gestaltung und Illustration<br />
Basel, Switzerland<br />
Printed by<br />
Druckerei Lüdin AG<br />
Liestal, Switzerland<br />
Products of <strong>Synthes</strong>, Inc. are covered by numerous<br />
trademark registration in various jurisdictions<br />
and by nearly 200 patents worldwide. <strong>Synthes</strong> is a<br />
registered trademark.<br />
60
www.synthes.com<br />
USA and Canada<br />
<strong>Synthes</strong>, Inc.<br />
1302 Wrights Lane East<br />
West Chester, PA 19380<br />
USA<br />
Tel. +1 610 719 5000<br />
US Customer Service (toll free):<br />
Tel. +1 800 523 0322<br />
Europe, Middle East and Africa<br />
<strong>Synthes</strong> GmbH<br />
Glutz Blotzheim-Str. 3<br />
4500 Solothurn<br />
Switzerland<br />
Tel. +41 32 720 40 60<br />
Fax +41 32 720 40 61<br />
ir.info@synthes.com<br />
Asia Pacific<br />
<strong>Synthes</strong> Asia Pacific<br />
Suite 1a<br />
Building 3, Level 3<br />
20 Bridge Street<br />
Pymble, NSW 2073<br />
Australia<br />
Tel. +61 2 9449 0400<br />
Fax +61 2 9449 0499<br />
Latin America<br />
<strong>Synthes</strong> LAT, Inc.<br />
703 N.W. 62nd Ave.<br />
Suite 550<br />
Miami, FL 33126<br />
USA<br />
Tel. +1 305 341 1022<br />
Fax +1 305 341 1028<br />
synthes.lat@synthes.com<br />
035.000.164 1/08 © <strong>Synthes</strong>, Inc. Printed in Switzerland