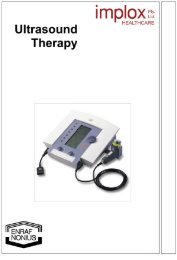
Directions for Use - Implox
Directions for Use - Implox
Directions for Use - Implox
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Laerdal Medical<br />
About this edition<br />
The in<strong>for</strong>mation in this guide applies to the<br />
model M5067A HeartStart Defibrillator. Its<br />
technical contents apply to all models in the<br />
HeartStart HS1 family of defibrillators,<br />
including the HeartStart, the HeartStart<br />
OnSite, and the HeartStart First Aid<br />
Defibrillator. This in<strong>for</strong>mation is subject to<br />
change. Please contact Laerdal <strong>for</strong><br />
in<strong>for</strong>mation on revisions.<br />
Edition history<br />
Edition 7<br />
Publication date: July 2007<br />
Publication #: M5067-91901<br />
Assembly #: 011826-0007<br />
Printed in the U.S.A.<br />
Copyright<br />
© 2007 Philips Electronics North America<br />
Corp.<br />
No part of this publication may be<br />
reproduced, transmitted, transcribed,<br />
stored in a retrieval system or translated<br />
into any human or computer language in any<br />
<strong>for</strong>m by any means without the consent of<br />
the copyright holder.<br />
Unauthorized copying of this publication<br />
may not only infringe copyright but also<br />
reduce the ability of Philips Medical Systems<br />
to provide accurate and up-to-date<br />
in<strong>for</strong>mation to users and operators alike.<br />
Authorized EU representative<br />
Philips Medizin Systeme Boeblingen GmbH<br />
Hewlett-Packard Strasse 2<br />
71034 Boeblingen, Germany<br />
(+49) 7031 463-2254<br />
CAUTION: Federal law (USA) restricts this device to sale<br />
by or on the order of a physician.<br />
The HeartStart Defibrillator is designed to be used only<br />
with Laerdal-approved accessories. The HeartStart may<br />
per<strong>for</strong>m improperly if non-approved accessories are used.<br />
Device tracking<br />
In the U.S.A., this device is subject to tracking requirements<br />
by the manufacturer and distributors. If the defibrillator has<br />
been sold, donated, lost, stolen, exported, or destroyed,<br />
notify Philips Medical Systems or your distributor.<br />
Device manufacturer<br />
The HeartStart Defibrillator is manufactured by Philips<br />
Medical Systems, Seattle, Washington, USA.<br />
Patents<br />
This product is manufactured and sold under one or more<br />
of the following United States patents: US6047212,<br />
US6317635, US5892046, US5891049, US6356785,<br />
US5650750, US6553257, US5902249, US6287328,<br />
US6662056, US5617853, US5951598, US6272385,<br />
US6234816, US6346014, US6230054,<br />
US6299574, US5607454, US5803927, US5735879,<br />
US5749905, US5601612, US6441582, US5889388,<br />
US5773961, US6016059, US6075369, US5904707,<br />
US5868792, US5899926, US5879374, US5632280,<br />
US5800460, US6185458, US5611815, US6556864,<br />
US5607454, and other patents pending.<br />
HEARTSTART M5067A