Osmo-dehydration of fruits
Osmo-dehydration of fruits
Osmo-dehydration of fruits
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
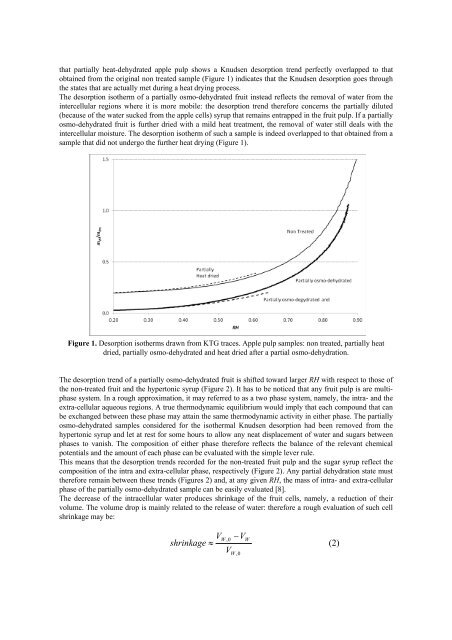
that partially heat-dehydrated apple pulp shows a Knudsen desorption trend perfectly overlapped to that<br />
obtained from the original non treated sample (Figure 1) indicates that the Knudsen desorption goes through<br />
the states that are actually met during a heat drying process.<br />
The desorption isotherm <strong>of</strong> a partially osmo-dehydrated fruit instead reflects the removal <strong>of</strong> water from the<br />
intercellular regions where it is more mobile: the desorption trend therefore concerns the partially diluted<br />
(because <strong>of</strong> the water sucked from the apple cells) syrup that remains entrapped in the fruit pulp. If a partially<br />
osmo-dehydrated fruit is further dried with a mild heat treatment, the removal <strong>of</strong> water still deals with the<br />
intercellular moisture. The desorption isotherm <strong>of</strong> such a sample is indeed overlapped to that obtained from a<br />
sample that did not undergo the further heat drying (Figure 1).<br />
Figure 1. Desorption isotherms drawn from KTG traces. Apple pulp samples: non treated, partially heat<br />
dried, partially osmo-dehydrated and heat dried after a partial osmo-<strong>dehydration</strong>.<br />
The desorption trend <strong>of</strong> a partially osmo-dehydrated fruit is shifted toward larger RH with respect to those <strong>of</strong><br />
the non-treated fruit and the hypertonic syrup (Figure 2). It has to be noticed that any fruit pulp is are multiphase<br />
system. In a rough approximation, it may referred to as a two phase system, namely, the intra- and the<br />
extra-cellular aqueous regions. A true thermodynamic equilibrium would imply that each compound that can<br />
be exchanged between these phase may attain the same thermodynamic activity in either phase. The partially<br />
osmo-dehydrated samples considered for the isothermal Knudsen desorption had been removed from the<br />
hypertonic syrup and let at rest for some hours to allow any neat displacement <strong>of</strong> water and sugars between<br />
phases to vanish. The composition <strong>of</strong> either phase therefore reflects the balance <strong>of</strong> the relevant chemical<br />
potentials and the amount <strong>of</strong> each phase can be evaluated with the simple lever rule.<br />
This means that the desorption trends recorded for the non-treated fruit pulp and the sugar syrup reflect the<br />
composition <strong>of</strong> the intra and extra-cellular phase, respectively (Figure 2). Any partial <strong>dehydration</strong> state must<br />
therefore remain between these trends (Figures 2) and, at any given RH, the mass <strong>of</strong> intra- and extra-cellular<br />
phase <strong>of</strong> the partially osmo-dehydrated sample can be easily evaluated [8].<br />
The decrease <strong>of</strong> the intracellular water produces shrinkage <strong>of</strong> the fruit cells, namely, a reduction <strong>of</strong> their<br />
volume. The volume drop is mainly related to the release <strong>of</strong> water: therefore a rough evaluation <strong>of</strong> such cell<br />
shrinkage may be:<br />
VW<br />
,0<br />
−VW<br />
shrinkage ≈ (2)<br />
V<br />
W ,0