9 ALDEHYDES AND KETONES
9 ALDEHYDES AND KETONES
9 ALDEHYDES AND KETONES
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
9 <strong>ALDEHYDES</strong> <strong>AND</strong> <strong>KETONES</strong><br />
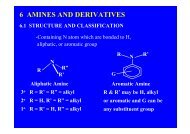
9.1 STRUCTURES <strong>AND</strong> CLASSIFICATION<br />
-Carbonyl compounds are classified as aldehydes and ketones both<br />
containing carbonyl group<br />
O<br />
R<br />
C<br />
H<br />
Aldehydes<br />
O<br />
R<br />
C<br />
R’<br />
Ketones<br />
R and R' may be aliphatic or aromatic
9.2 NOMENCLATURE<br />
(CH 3 ) 2 CHCHO CH 3 CH 2 COCH 3<br />
Isobutyraldehyde<br />
Ethyl methyl ketone -Common name<br />
2-Methylpropanal 2-Butanone -IUPAC name<br />
CHO<br />
CHO<br />
COCH 3<br />
CH 3<br />
Cyclohexanecarboxaldehyde<br />
Cyclohexanecarbaldehyde<br />
p-Tolualdehyde Acetophenone<br />
4-Methylbenzaldehyde Phenylethanone
9.3 PHYSICAL PROPERTIES<br />
-polar<br />
-moderate boiling lines between ethers and<br />
-small molecules are water soluble<br />
alcohols<br />
9.4 INDUSTRIAL SOURCES<br />
-Natural source → Carbohydrates, Cinnamon, Vanilla,<br />
Peppermint, Camphor<br />
-From synthesis → from alcohols (1 o and 2 o )<br />
-Mainly used as solvents, reagents, preservatives
9.5 TYPICAL REACTIONS<br />
-Addition of Organometallic Reagents<br />
OH<br />
CH<br />
CH 3 CHO ⎯⎯⎯⎯⎯⎯→ 3 MgI/Et 2 O aq.NH<br />
⎯⎯⎯⎯→ 4 Cl<br />
CH 3 CH-CH 3<br />
-Addition of Cyanides<br />
OH<br />
C 6 H 5 CHO + NaCN/H 2 O ⎯→ C 6 H 5 CH-CN<br />
-Addition of Alcohols<br />
C 6 H 5 CHO + 2CH 3 OH/HCl → C 6 H 5 CH(OCH 3 ) 2 + H 2 O<br />
-Addition of Amines and Derivatives of Ammonia<br />
CH 3 CHO + CH 3 NH 2 /H 3 O + ⎯→ CH 3 CH=NCH 3
O<br />
N-OH<br />
+ HO-NH 3+ Cl - ⎯→<br />
-Cannizzaro Reaction<br />
1) 60%KOH<br />
C 6 H 5 CHO ⎯⎯⎯⎯⎯→ C 6 H 5 COOH + C 6 H 5 CH 2 OH<br />
2) H 3 O +<br />
-Oxidation of Aldehydes<br />
CH 3 CH 2 CH 2 CHO + KMnO 4 → CH 3 CH 2 CH 2 COOH<br />
2Ag(NH 3 )<br />
+<br />
2<br />
CH 3 CH=CHCHO ⎯⎯⎯⎯→ CH 3 CH=CHCOO - + 2Ag + 4NH 3 +<br />
3OH -<br />
2H 2 O
-Reduction to Alcohols<br />
OH<br />
1) LiAlH<br />
C 6 H 5 COCH 3 ⎯⎯⎯⎯⎯⎯→ 4 /Et 2 O<br />
C 6 H 5 CH-CH 3<br />
2) H 3 O +<br />
-Reduction to Hydrocarbons<br />
H 2 NNH 2 /DMSO<br />
(C 6 H 5 ) 2 CO ⎯⎯⎯⎯⎯⎯→ (C 6 H 5 ) 2 CH 2 (90%)<br />
t-BuOK/t-BuOH<br />
Zn(Hg)/HCl<br />
C 6 H 5 COCH 3 ⎯⎯⎯⎯⎯→ C 6 H 5 CH 2 CH 3<br />
heat
-Halogenation<br />
CH 3 CH 2 COCH 3 + Br 2 /aq.HCl ⎯→ CH 3 CH 2 COCH 2 Br<br />
-Addition of Carbanions<br />
HO CH<br />
aq.NaOH/10<br />
2CH 3 CH 2 CHO ⎯⎯⎯⎯⎯⎯⎯→ o 3<br />
C<br />
CH 3 CH 2 CH-CH-CHO<br />
-Electrophilic Aromatic Substitution<br />
COCH 3 COCH 3<br />
+ HNO 3 /H 2 SO 4 ⎯→<br />
NO 2
9.6 LABORATORY PREPARATIONS<br />
-From oxidation of alcohols<br />
-From acyl chlorides<br />
-From aromatic hydrocarbons<br />
9.7 ANALYSIS OF CARBONYL COMPOUNDS<br />
-Form yellow or red precipitate with ammonium derivatives<br />
-Oxidized by cold dil.neutral KMnO 4<br />
or CrO 3<br />
/H 2<br />
SO 4<br />
-Aldehydes are distinguished from ketones by mild oxidation:<br />
Tollens reagent or Benedict’s solution or Fehling’s solution<br />
-Schiff test (Fuchsin-aldehyde reagent) gives reddish violet color<br />
-Methyl ketones (Acetaldehyde) give positive iodoform test