Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
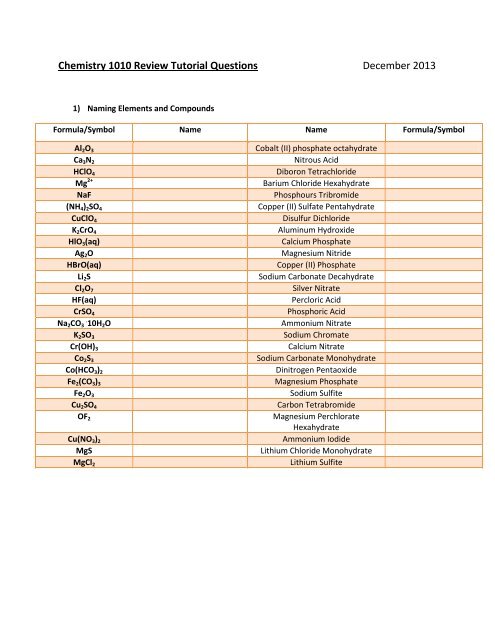
<strong>Chemistry</strong> 1010 Review Tutorial Questions December 2013<br />
1) Naming Elements and Compounds<br />
Formula/Symbol Name Name Formula/Symbol<br />
Al 2 O 3<br />
Ca 3 N 2<br />
HClO 4<br />
Mg 2+<br />
NaF<br />
(NH 4 ) 2 SO 4<br />
CuClO 4<br />
K 2 CrO 4<br />
HlO 3 (aq)<br />
Ag 2 O<br />
HBrO(aq)<br />
Li 2 S<br />
Cl 2 O 7<br />
HF(aq)<br />
CrSO 4<br />
Na 2 CO . 3 10H 2 O<br />
K 2 SO 3<br />
Cr(OH) 3<br />
Co 2 S 3<br />
Co(HCO 3 ) 2<br />
Fe 2 (CO 3 ) 3<br />
Fe 2 O 3<br />
Cu 2 SO 4<br />
OF 2<br />
Cu(NO 3 ) 2<br />
MgS<br />
MgCl 2<br />
Cobalt (II) phosphate octahydrate<br />
Nitrous Acid<br />
Diboron Tetrachloride<br />
Barium Chloride Hexahydrate<br />
Phosphours Tribromide<br />
Copper (II) Sulfate Pentahydrate<br />
Disulfur Dichloride<br />
Aluminum Hydroxide<br />
Calcium Phosphate<br />
Magnesium Nitride<br />
Copper (II) Phosphate<br />
Sodium Carbonate Decahydrate<br />
Silver Nitrate<br />
Percloric Acid<br />
Phosphoric Acid<br />
Ammonium Nitrate<br />
Sodium Chromate<br />
Calcium Nitrate<br />
Sodium Carbonate Monohydrate<br />
Dinitrogen Pentaoxide<br />
Magnesium Phosphate<br />
Sodium Sulfite<br />
Carbon Tetrabromide<br />
Magnesium Perchlorate<br />
Hexahydrate<br />
Ammonium Iodide<br />
Lithium Chloride Monohydrate<br />
Lithium Sulfite
2) Balancing Chemical Equations<br />
__N 2 O 5 + __H 2 O → __HNO 3<br />
__MnO 2 + __HCl → __MnCl 2 + __Cl 2 + __H 2 O<br />
__Na 2 S 2 O 3 + __I 2 → __NaI + __Na 2 S 4 O 6<br />
__Al 4 C 3 + __H 2 O → __Al(OH) 3 + __CH 4<br />
__Ca 3 (PO 4 ) 2 + __C → __Ca 3 P 2 + __CO<br />
3) Predict whether the following compounds are soluble in water<br />
a) PbSO 4<br />
b) KNO 3<br />
c) Ca(CH 3 COO) 2<br />
d) Hg 2 Cl 2<br />
e) SrCO 3<br />
f) LiCl<br />
4) Write molecular, complete and net ionic equations for the following reactions. Also, classify<br />
each reaction as precipitation, redox , acid/base, gas evolution or no reaction:<br />
a) When Ca metal and hydrochloric acid are mixed<br />
b) A solution of aqueous Barium Hydroxide is mixed with Nitric Acid<br />
c) Aqueous Sodium Carbonate reacts with Hydrochloric Acid<br />
d) Aqueous Barium Chloride reacts with Sodium Sulfate<br />
e) Solid zinc reacts with Hydrochloric acid<br />
f) Aqueous Silver Nitrate reacts with aqueous Magnesium Chloride<br />
g) Aqueous solutions of Lead (II) nitrate and Sodium Chloride<br />
h) Solutions of Sodium Phosphate and Iron (II) Chloride<br />
i) Ca(s) + AgNO 3 -><br />
j) HNO 3 (aq) + Ba(OH) 2 (aq) -><br />
k) Pb(NO 3 ) 2 (aq) + NaI (aq) -><br />
Write molecular, complete and net ionic equations for the following reactions.<br />
a) Aqueous Cobalt (II) Chloride reacts with aqueous Silver Nitrate
) Nitric Acid reacts with aqueous Calcium Hydroxide<br />
c) Aluminum Metal reacts with aqueous Coper (II) Chloride<br />
d) Solutions of Copper (II) Chloride reacts with Silver Nitrate<br />
e) Zinc solid reacts with Copper (II) Sulfate<br />
f) Ammonia solution reacts with Hydrochloric Acid<br />
g) The reaction between Sodium metal and Water<br />
h) The combustion of Methane (CH4) gas in Oxygen<br />
i) Neutralization reaction between Sulfuric Acid and aqueous Potassium Hydroxide<br />
j) Chromium (III) Chloride solution reacts with Sodium Sulfide to give a black ppt.<br />
k) Silver Nitrate solution reacts with Sodium Chloride to give a white ppt.<br />
l) Ammonium Phosphate solution reacts with Nickel (II) Chloride<br />
m) Aqueous Barium Hydroxide reacts with Nitric Acid<br />
n) Aqueous Chromium (III) Sulfate reacts with aqueous Ammonium Carbonate<br />
o) Aqueous Phosphoric Acid reacts with aqueous Calcium Hydroxide<br />
p) Solutions of Copper (II) Sulfate reacts with Strontium Nitrate (Sr(NO3)2)<br />
q) A strip of Zinc is added to a solution of Lead (II) Nitrate<br />
5) Assign oxidation numbers to each of the atoms indicated<br />
a) Nb in Nb 2 O 5<br />
b) P in HPO 4<br />
2–<br />
c) Ga in Ga(H 2 PO 4 ) 3<br />
d) N in N 2 H 5<br />
+<br />
e) Cl in Cl 2(g)<br />
f) Fe 3+<br />
g) C in CH 2 Cl 2<br />
6) Determine whether each of the following is a redox reaction by assigning oxidation numbers.<br />
If it is, identify the oxidizing and reducing agents:<br />
a) CuCl 2(aq) + Al (s) → AlCl 3(aq) + Cu (s)<br />
b) KSO 4(aq) + Ba(NO 3 ) 2(aq) → BaSO 4(s) + KNO 3(aq)<br />
c) Na (s) + Au(NO 3 ) 3(aq) → NaNO 3(aq) + Au (s)
7) Balance the following Redox Reactions under acidic and basic conditions.<br />
a) C 4 H 4 O - 6 (aq) + ClO - 3 (aq) CO - 3 (aq) + Cl - (aq)<br />
-<br />
b) H 2 O 2 (aq) + Cl 2 O 7 (aq) ClO 2 (aq) + O 2 (g)<br />
c) Tl 2 O 3 (aq) + NH 2 OH TlOH (s) + N 2 (g)<br />
d) Mn(OH) 2 (s) + MnO - 4 (aq) MnO 2 (s)<br />
2-<br />
e) S 4 O 6 (aq) + Al (s) H 2 S (aq) + Al 3+ (aq)