Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
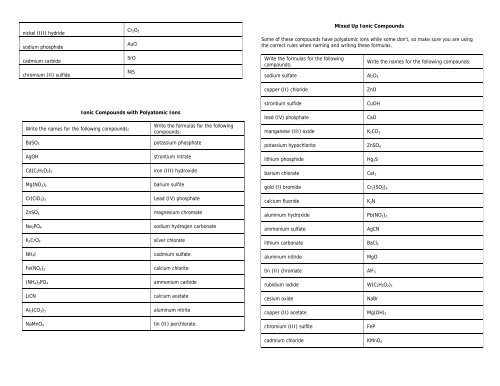
nickel (III) hydride<br />
sodium phosphide<br />
cadmium carbide<br />
chromium (II) sulfide<br />
Cr 2O 3<br />
AuCl<br />
SrO<br />
NiS<br />
Mixed Up <strong>Ionic</strong> <strong>Compounds</strong><br />
Some of these compounds have polyatomic ions while some don’t, so make sure you are using<br />
the correct rules when <strong>naming</strong> and writing these formulas.<br />
Write the formulas for the following<br />
compounds:<br />
sodium sulfate Al 2O 3<br />
Write the names for the following compounds:<br />
copper (II) chloride<br />
ZnO<br />
Write the names for the following compounds:<br />
<strong>Ionic</strong> <strong>Compounds</strong> with Polyatomic Ions<br />
Write the formulas for the following<br />
compounds:<br />
strontium sulfide<br />
CuOH<br />
lead (IV) phosphate<br />
CaO<br />
manganese (III) oxide K 2CO 3<br />
BaSO 3<br />
potassium phosphate<br />
potassium hypochlorite ZnSO 4<br />
AgOH<br />
strontium nitrate<br />
lithium phosphide<br />
Hg 2S<br />
Cd(C 2H 3O 2) 2<br />
Mg(NO 3) 2<br />
iron (III) hydroxide<br />
barium sulfite<br />
barium chlorate CaI 2<br />
gold (I) bromide Cr 2(SO 3) 3<br />
Cr(ClO 3) 3<br />
Lead (IV) phosphate<br />
calcium fluoride<br />
K 3N<br />
ZnSO 4<br />
magnesium chromate<br />
aluminum hydroxide Pb(NO 3) 2<br />
Na 3PO 4<br />
sodium hydrogen carbonate<br />
ammonium sulfate<br />
AgCN<br />
K 2CrO 4<br />
silver chlorate<br />
lithium carbonate BaCl 2<br />
NH 4I<br />
cadmium sulfate<br />
aluminum nitride<br />
MgO<br />
Fe(NO 2) 2<br />
(NH 4) 3PO 4<br />
calcium chlorite<br />
ammonium carbide<br />
tin (II) chromate AlF 3<br />
rubidium iodide W(C 2H 3O 2) 5<br />
LiCN<br />
calcium acetate<br />
cesium oxide<br />
NaBr<br />
Al 2(CO 3) 3<br />
aluminum nitrite<br />
copper (II) acetate Mg(OH) 2<br />
NaMnO 4<br />
tin (II) perchlorate<br />
chromium (III) sulfite<br />
FeP<br />
cadmium chloride KMnO 4