prior authorization/notification list for inet and onet ... - Health Net
prior authorization/notification list for inet and onet ... - Health Net
prior authorization/notification list for inet and onet ... - Health Net
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
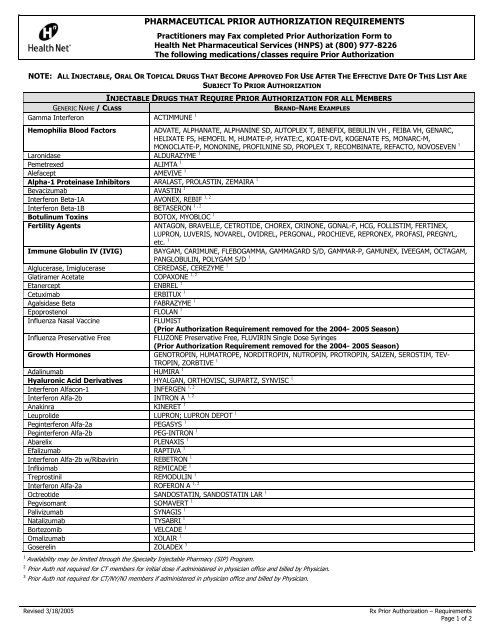
PHARMACEUTICAL PRIOR AUTHORIZATION REQUIREMENTS<br />
Practitioners may Fax completed Prior Authorization Form to<br />
<strong>Health</strong> <strong>Net</strong> Pharmaceutical Services (HNPS) at (800) 977-8226<br />
The following medications/classes require Prior Authorization<br />
NOTE: ALL INJECTABLE, ORAL OR TOPICAL DRUGS THAT BECOME APPROVED FOR USE AFTER THE EFFECTIVE DATE OF THIS LIST ARE<br />
SUBJECT TO PRIOR AUTHORIZATION<br />
INJECTABLE DRUGS THAT REQUIRE PRIOR AUTHORIZATION FOR ALL MEMBERS<br />
GENERIC NAME / CLASS<br />
BRAND-NAME EXAMPLES<br />
Gamma Interferon ACTIMMUNE 1<br />
Hemophilia Blood Factors<br />
ADVATE, ALPHANATE, ALPHANINE SD, AUTOPLEX T, BENEFIX, BEBULIN VH , FEIBA VH, GENARC,<br />
HELIXATE FS, HEMOFIL M, HUMATE-P, HYATE:C, KOATE-DVI, KOGENATE FS, MONARC-M,<br />
MONOCLATE-P, MONONINE, PROFILNINE SD, PROPLEX T, RECOMBINATE, REFACTO, NOVOSEVEN 1<br />
Laronidase ALDURAZYME 1<br />
Pemetrexed ALIMTA 1<br />
Alefacept AMEVIVE 1<br />
Alpha-1 Proteinase Inhibitors ARALAST, PROLASTIN, ZEMAIRA 1<br />
Bevacizumab AVASTIN 1<br />
Interferon Beta-1A AVONEX, REBIF 1, 2<br />
Interferon Beta-1B BETASERON 1 , 2<br />
Botulinum Toxins BOTOX, MYOBLOC 1<br />
Fertility Agents<br />
ANTAGON, BRAVELLE, CETROTIDE, CHOREX, CRINONE, GONAL-F, HCG, FOLLISTIM, FERTINEX,<br />
LUPRON, LUVERIS, NOVAREL, OVIDREL, PERGONAL, PROCHIEVE, REPRONEX, PROFASI, PREGNYL,<br />
etc. 1<br />
Immune Globulin IV (IVIG) BAYGAM, CARIMUNE, FLEBOGAMMA, GAMMAGARD S/D, GAMMAR-P, GAMUNEX, IVEEGAM, OCTAGAM,<br />
PANGLOBULIN, POLYGAM S/D 1<br />
Alglucerase, Imiglucerase CEREDASE, CEREZYME 1<br />
Glatiramer Acetate COPAXONE 1, 2<br />
Etanercept ENBREL 1<br />
Cetuximab ERBITUX 1<br />
Agalsidase Beta FABRAZYME 1<br />
Epoprostenol FLOLAN 1<br />
Influenza Nasal Vaccine<br />
FLUMIST<br />
(Prior Authorization Requirement removed <strong>for</strong> the 2004- 2005 Season)<br />
Influenza Preservative Free<br />
FLUZONE Preservative Free, FLUVIRIN Single Dose Syringes<br />
(Prior Authorization Requirement removed <strong>for</strong> the 2004- 2005 Season)<br />
Growth Hormones<br />
GENOTROPIN, HUMATROPE, NORDITROPIN, NUTROPIN, PROTROPIN, SAIZEN, SEROSTIM, TEV-<br />
TROPIN, ZORBTIVE 1<br />
Adalinumab HUMIRA 1<br />
Hyaluronic Acid Derivatives HYALGAN, ORTHOVISC, SUPARTZ, SYNVISC 1<br />
Interferon Alfacon-1 INFERGEN 1, 2<br />
Interferon Alfa-2b INTRON A 1, 2<br />
Anakinra KINERET 1<br />
Leuprolide LUPRON; LUPRON DEPOT 1<br />
Peginterferon Alfa-2a PEGASYS 1<br />
Peginterferon Alfa-2b PEG-INTRON 1<br />
Abarelix PLENAXIS 1<br />
Efalizumab RAPTIVA 1<br />
Interferon Alfa-2b w/Ribavirin REBETRON 1<br />
Infliximab REMICADE 1<br />
Treprostinil REMODULIN 1<br />
Interferon Alfa-2a ROFERON A 1, 2<br />
Octreotide SANDOSTATIN, SANDOSTATIN LAR 1<br />
Pegvisomant SOMAVERT 1<br />
Palivizumab SYNAGIS 1<br />
Natalizumab TYSABRI 1<br />
Bortezomib VELCADE 1<br />
Omalizumab XOLAIR 1<br />
Goserelin ZOLADEX 3<br />
1<br />
Availability may be limited through the Specialty Injectable Pharmacy (SIP) Program.<br />
2 Prior Auth not required <strong>for</strong> CT members <strong>for</strong> initial dose if administered in physician office <strong>and</strong> billed by Physician.<br />
3 Prior Auth not required <strong>for</strong> CT/NY/NJ members if administered in physician office <strong>and</strong> billed by Physician.<br />
Revised 3/18/2005<br />
Rx Prior Authorization – Requirements<br />
Page 1 of 2
PHARMACEUTICAL PRIOR AUTHORIZATION REQUIREMENTS<br />
Practitioners may Fax completed Prior Authorization Form to<br />
<strong>Health</strong> <strong>Net</strong> Pharmaceutical Services (HNPS) at (800) 977-8226<br />
The following medications/classes require Prior Authorization<br />
DRUGS THAT REQUIRE PRIOR AUTHORIZATION FOR PRESCRIPTION PLANS<br />
NOT APPLICABLE TO MEDICAID HEALTHY OPTIONS PLANS<br />
SEE SEPARATE HEALTHY OPTIONS PRIOR AUTHORIZATION LIST<br />
DRUG CLASS / GENERIC NAME<br />
BRAND NAME EXAMPLES<br />
Compounded Prescriptions<br />
N/A<br />
COX-2 Inhibitors: Celecoxib, Etoricoxib,<br />
ARCOXIA, BEXTRA, CELEBREX, DYNASTAT, PREXIGE,<br />
Lumiracoxib, Parecoxib, Tilmacoxib, Valdecoxib<br />
Drugs used <strong>for</strong> Sexual Dysfunction <strong>for</strong> members ALISTA, ALPROX-TD, CAVERJECT, CIALIS, EDEX, INTRINSA, LEVITRA, MUSE, VIAGRA<br />
under age 40: Alprostadil, Tadalafil, Testosterone,<br />
Vardenafil, Sildenafil<br />
Medication Exceptions due to Drug Utilization See: QUANTITY LIMIT / DRUG UTILIZATION REVIEW List<br />
Review (DUR)<br />
Non-Sedating Antihistamines<br />
ALLEGRA, ALLEGRA-D, CLARINEX, ZYRTEC, ZYRTEC-D<br />
Cetirizine, Desloratadine, Fexofenadine<br />
Nutritional Supplements<br />
MSUD, PHENYL-FREE, XP-ANALOG, XPHEN, NEOCATE<br />
Onychomycosis Agents<br />
LAMISIL, PENLAC, SPORANOX<br />
(Not covered <strong>for</strong> cosmetic treatment):<br />
Ciclopirox, Itraconazole, Terbinafine<br />
Proton Pump Inhibitors:<br />
NEXIUM, PREVACID, PRILOSEC, PROTONIX, ZEGERID<br />
Esomeprazole, Lansoprazole, Omeprazole,<br />
Pantoprazole, Rabeprazole<br />
Testosterone Preparations<br />
ANDRODERM, ANDROGEL, STRIANT, TESTODERM, TESTIM, TOSTRELLE<br />
Topical Retinoids <strong>for</strong> members age 36 or older: AVITA, DIFFERIN, RETIN-A<br />
(Not covered <strong>for</strong> cosmetic use)<br />
Adapalene, Tretinoin<br />
Fentanyl Lozenge<br />
ACTIQ<br />
Apomorphine<br />
APOKYN<br />
Amlodipine / Atorvastatin<br />
CADUET<br />
Ribavirin COPEGUS, REBETOL, RIBASPHERE<br />
Duloxetine<br />
CYMBALTA<br />
Acamprosate<br />
CAMPRAL<br />
Fentanyl Patch<br />
DURAGESIC<br />
Inhaled Insulin<br />
EXUBERA<br />
Teriparatide<br />
FORTEO<br />
Enfuvirtide<br />
FUZEON<br />
Imatinib<br />
GLEEVEC<br />
Adefovir<br />
HEPSERA<br />
Eplerenone<br />
INSPRA<br />
Gefitinib<br />
IRESSA<br />
Eszopiclone<br />
LUNESTA<br />
Meloxicam<br />
MOBIC<br />
Oxycodone SR 12 HR<br />
OXYCONTIN<br />
Modafinil<br />
PROVIGIL<br />
Cyclosporine Ophthalmic<br />
RESTASIS<br />
Cinacalcet<br />
SENSIPAR<br />
Montelukast<br />
SINGULAIR<br />
Tiotropium<br />
SPIRIVA<br />
Erlotinib<br />
TARCEVA<br />
Temazolomide<br />
TEMODAR<br />
Thalidomide<br />
THALOMID<br />
Bosentan<br />
TRACLEER<br />
Sodium Oxybate<br />
XYREM<br />
Miglustat<br />
ZAVESCA<br />
HEALTH NET RESERVES THE RIGHT TO REQUIRE PRIOR AUTHORIZATION FOR ORAL AND TOPICAL DRUGS EXCEPT WHERE MANDATED BY LAW.<br />
Revised 3/18/2005<br />
Rx Prior Authorization – Requirements<br />
Page 2 of 2