LIFE01200604005 Shri Somnath Ghosh - Homi Bhabha National ...
LIFE01200604005 Shri Somnath Ghosh - Homi Bhabha National ...
LIFE01200604005 Shri Somnath Ghosh - Homi Bhabha National ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Bystander effect: role of iNOS d S. GHOSH et al. 1573<br />
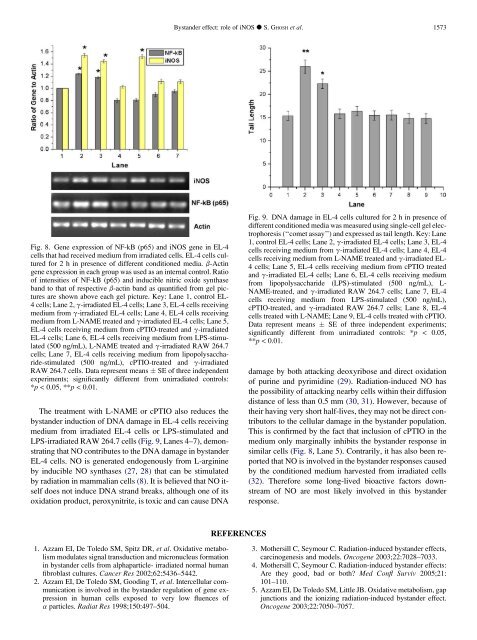
Fig. 8. Gene expression of NF-kB (p65) and iNOS gene in EL-4<br />
cells that had received medium from irradiated cells. EL-4 cells cultured<br />
for 2 h in presence of different conditioned media. b-Actin<br />
gene expression in each group was used as an internal control. Ratio<br />
of intensities of NF-kB (p65) and inducible nitric oxide synthase<br />
band to that of respective b-actin band as quantified from gel pictures<br />
are shown above each gel picture. Key: Lane 1, control EL-<br />
4 cells; Lane 2, g-irradiated EL-4 cells; Lane 3, EL-4 cells receiving<br />
medium from g-irradiated EL-4 cells; Lane 4, EL-4 cells receiving<br />
medium from L-NAME treated and g-irradiated EL-4 cells; Lane 5,<br />
EL-4 cells receiving medium from cPTIO-treated and g-irradiated<br />
EL-4 cells; Lane 6, EL-4 cells receiving medium from LPS-stimulated<br />
(500 ng/mL), L-NAME treated and g-irradiated RAW 264.7<br />
cells; Lane 7, EL-4 cells receiving medium from lipopolysaccharide-stimulated<br />
(500 ng/mL), cPTIO-treated and g-irradiated<br />
RAW 264.7 cells. Data represent means SE of three independent<br />
experiments; significantly different from unirradiated controls:<br />
*p < 0.05, **p < 0.01.<br />
The treatment with L-NAME or cPTIO also reduces the<br />
bystander induction of DNA damage in EL-4 cells receiving<br />
medium from irradiated EL-4 cells or LPS-stimulated and<br />
LPS-irradiated RAW 264.7 cells (Fig. 9, Lanes 4–7), demonstrating<br />
that NO contributes to the DNA damage in bystander<br />
EL-4 cells. NO is generated endogenously from L-arginine<br />
by inducible NO synthases (27, 28) that can be stimulated<br />
by radiation in mammalian cells (8). It is believed that NO itself<br />
does not induce DNA strand breaks, although one of its<br />
oxidation product, peroxynitrite, is toxic and can cause DNA<br />
Fig. 9. DNA damage in EL-4 cells cultured for 2 h in presence of<br />
different conditioned media was measured using single-cell gel electrophoresis<br />
(‘‘comet assay’’) and expressed as tail length. Key: Lane<br />
1, control EL-4 cells; Lane 2, g-irradiated EL-4 cells; Lane 3, EL-4<br />
cells receiving medium from g-irradiated EL-4 cells; Lane 4, EL-4<br />
cells receiving medium from L-NAME treated and g-irradiated EL-<br />
4 cells; Lane 5, EL-4 cells receiving medium from cPTIO treated<br />
and g-irradiated EL-4 cells; Lane 6, EL-4 cells receiving medium<br />
from lipopolysaccharide (LPS)-stimulated (500 ng/mL), L-<br />
NAME-treated, and g-irradiated RAW 264.7 cells; Lane 7, EL-4<br />
cells receiving medium from LPS-stimulated (500 ng/mL),<br />
cPTIO-treated, and g-irradiated RAW 264.7 cells; Lane 8, EL-4<br />
cells treated with L-NAME; Lane 9, EL-4 cells treated with cPTIO.<br />
Data represent means SE of three independent experiments;<br />
significantly different from unirradiated controls: *p < 0.05,<br />
**p < 0.01.<br />
damage by both attacking deoxyribose and direct oxidation<br />
of purine and pyrimidine (29). Radiation-induced NO has<br />
the possibility of attacking nearby cells within their diffusion<br />
distance of less than 0.5 mm (30, 31). However, because of<br />
their having very short half-lives, they may not be direct contributors<br />
to the cellular damage in the bystander population.<br />
This is confirmed by the fact that inclusion of cPTIO in the<br />
medium only marginally inhibits the bystander response in<br />
similar cells (Fig. 8, Lane 5). Contrarily, it has also been reported<br />
that NO is involved in the bystander responses caused<br />
by the conditioned medium harvested from irradiated cells<br />
(32). Therefore some long-lived bioactive factors downstream<br />
of NO are most likely involved in this bystander<br />
response.<br />
REFERENCES<br />
1. Azzam EI, De Toledo SM, Spitz DR, et al. Oxidative metabolism<br />
modulates signal transduction and micronucleus formation<br />
in bystander cells from alphaparticle- irradiated normal human<br />
fibroblast cultures. Cancer Res 2002;62:5436–5442.<br />
2. Azzam EI, De Toledo SM, Gooding T, et al. Intercellular communication<br />
is involved in the bystander regulation of gene expression<br />
in human cells exposed to very low fluences of<br />
a particles. Radiat Res 1998;150:497–504.<br />
3. Mothersill C, Seymour C. Radiation-induced bystander effects,<br />
carcinogenesis and models. Oncogene 2003;22:7028–7033.<br />
4. Mothersill C, Seymour C. Radiation-induced bystander effects:<br />
Are they good, bad or both? Med Confl Surviv 2005;21:<br />
101–110.<br />
5. Azzam EI, De Toledo SM, Little JB. Oxidative metabolism, gap<br />
junctions and the ionizing radiation-induced bystander effect.<br />
Oncogene 2003;22:7050–7057.