Osteoporosis guidelines (21 July 2011) - Hampshire Hospitals NHS ...
Osteoporosis guidelines (21 July 2011) - Hampshire Hospitals NHS ...
Osteoporosis guidelines (21 July 2011) - Hampshire Hospitals NHS ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
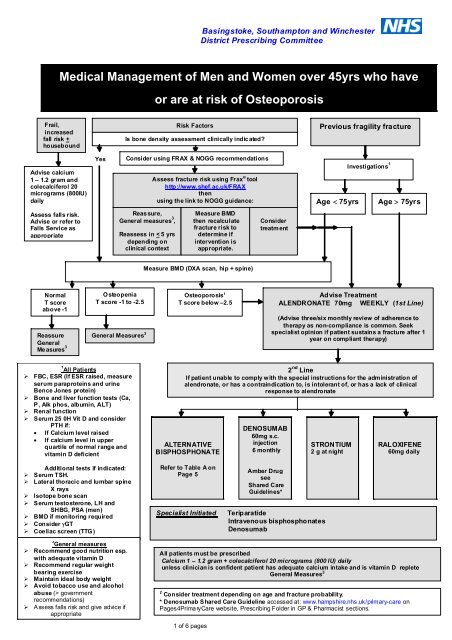
Basingstoke, Southampton and Winchester<br />
District Prescribing Committee<br />
Medical Management of Men and Women over 45yrs who have<br />
or are at risk of <strong>Osteoporosis</strong><br />
Frail,<br />
increased<br />
fall risk +<br />
housebound<br />
Risk Factors<br />
Is bone density assessment clinically indicated?<br />
Previous fragility fracture<br />
Advise calcium<br />
1 – 1.2 gram and<br />
colecalciferol 20<br />
micrograms (800IU)<br />
daily<br />
Yes<br />
Consider using FRAX & NOGG recommendations<br />
Assess fracture risk using Frax ® tool<br />
http://www.shef.ac.uk/FRAX<br />
then<br />
using the link to NOGG guidance:<br />
Investigations 1<br />
Age 75yrs Age 75yrs<br />
Assess falls risk.<br />
Advise or refer to<br />
Falls Service as<br />
appropriate<br />
Reas sure,<br />
General measures 3 ,<br />
Reassess in < 5 yrs<br />
depending on<br />
clinical context<br />
Measure BMD<br />
then recalculate<br />
fracture risk to<br />
determine if<br />
intervention is<br />
appropriate.<br />
Consider<br />
treatment<br />
Measure BMD (DXA scan, hip + spine)<br />
Normal<br />
T score<br />
‣ above -1<br />
Osteopenia<br />
T score -1 to -2.5<br />
Reassure General Measures 3<br />
General<br />
Me asures 3<br />
<strong>Osteoporosis</strong> 2<br />
T score below –2.5<br />
Advise Treatment<br />
ALENDRONATE 70mg WEEKLY (1st Line)<br />
(Advise three/six monthly review of adherence to<br />
therapy as non-compliance is common. Seek<br />
specialist opinion if patient sustains a fracture after 1<br />
year on compliant therapy)<br />
1<br />
All Patients<br />
‣ FBC, ESR (If ESR raised, measure<br />
serum paraproteins and urine<br />
Bence Jones protein)<br />
‣ Bone and liver function tests (Ca,<br />
P, Alk phos, albumin, ALT)<br />
‣ Renal function<br />
‣ Serum 25 0H Vit D and consider<br />
PTH if:<br />
If Calcium level raised<br />
If calcium level in upper<br />
quartile of normal range and<br />
vitamin D deficient<br />
2 nd Line<br />
If patient unable to comply with the special instructions for the administration of<br />
alendronate, or has a contraindication to, is intolerant of, or has a lack of clinical<br />
response to alendronate<br />
ALTERNATIVE<br />
BISPHOSPHONATE<br />
DENOSUMAB<br />
60mg s.c.<br />
injection<br />
6 monthly<br />
STRONTIUM<br />
2 g at night<br />
RALOXIFENE<br />
60mg daily<br />
Additional tests if indicated:<br />
‣ Serum TSH.<br />
‣ Lateral thoracic and lumbar spine<br />
X rays<br />
‣ Isotope bone scan<br />
‣ Serum testosterone, LH and<br />
SHBG, PSA (men)<br />
‣ BMD if monitoring required<br />
‣ Consider GT<br />
‣ Coeliac screen (TTG)<br />
Refer to Table A on<br />
Page 5<br />
Specialist Initiated<br />
Amber Drug<br />
see<br />
Shared Care<br />
Guidelines*<br />
Teriparatide<br />
Intravenous bisphosphonates<br />
Denosumab<br />
3<br />
General measures<br />
‣ Recommend good nutrition esp.<br />
with adequate vitamin D<br />
‣ Recommend regular weight<br />
bearing exercise<br />
‣ Maintain ideal body weight<br />
‣ Avoid tobacco use and alcohol<br />
abuse (> government<br />
recommendations)<br />
‣ A ssess falls risk and give advice if<br />
appropriate<br />
All patients must be prescribed<br />
Calcium 1 – 1.2 gram + colecalciferol 20 micrograms (800 IU) daily<br />
unless clinician is confident patient has adequate calcium intake and is vitamin D replete<br />
General Measures 3<br />
2 Consider treatment depending on age and fracture probability.<br />
* Denosumab Shared Care Guideline accessed at: www.hampshire.nhs.uk/primary-care on<br />
Pages4PrimaryCare website, Prescribing Folder in GP & Pharmacist sections.<br />
1 of 6 pages
Basingstoke, Southampton and Winchester<br />
District Prescribing Committee<br />
ALGORITHM FOR THE MEDICAL MANAGEMENT OF GLUCOCORTICOID-INDUCED<br />
OSTEOPOROSIS IN ADULTS<br />
Glucocorticoid therapy expected to be 3months<br />
or<br />
Cumulative dose equivalent to 1.5gram per year for<br />
patients prescribed repeated short courses)<br />
Age 65yrs<br />
Age 65yrs<br />
No<br />
previous<br />
fragility<br />
fracture<br />
Previous fragility<br />
fracture or incident<br />
fracture<br />
Investigations 1<br />
Measure BMD<br />
(DXA scan, hip + spine<br />
T score above 0<br />
Reassure<br />
General<br />
measures 3<br />
Repeat BMD<br />
not indicated<br />
unless a daily<br />
dose of 10mg<br />
or more is<br />
required<br />
T score between<br />
0 and - 1.5<br />
General<br />
measures 3<br />
Repeat BMD in<br />
1 – 3 yr if<br />
glucocorticoids<br />
continued<br />
T score<br />
– 1.5 or lower 2 Treatment<br />
RISEDRONATE 35mg WEEKLY<br />
(or other bisphosphonate if not<br />
tolerated)<br />
All patients must also be prescribed:<br />
Calcium 1 – 1.2 gram + colecalciferol 20 micrograms<br />
(800 IU) daily<br />
unless clinician is confident patient has adequate<br />
calcium intake and is vitamin D replete<br />
Initiate osteoporosis management when<br />
glucocorticoid is started and stop treatment six<br />
months after glucocorticoids stop.<br />
Advise three/six monthly review of<br />
adherence to therapy.<br />
1<br />
All Patients<br />
‣ FBC, ESR (If ESR raised, measure serum<br />
paraproteins and urine Bence Jones protein)<br />
‣ Bone and liver function tests (Ca, P, Alk phos,<br />
albumin, ALT)<br />
‣ Renal function<br />
‣ Serum 25 0H Vit D and consider PTH if:<br />
If Calcium level raised<br />
If calcium level in upper quartile of<br />
normal range and vitamin D deficient<br />
Additional tests if indicated:<br />
‣ Serum TSH.<br />
‣ Serum 25 0H Vit D<br />
‣ Lateral thoracic and lumbar spine X rays<br />
‣ Isotope bone scan<br />
‣ Serum testosterone, LH and SHBG, PSA (men)<br />
‣ BMD if monitoring required<br />
‣ Consider GT<br />
‣ Coeliac screen (TTG)<br />
2<br />
Consider treatment depending on age and fracture probability<br />
3<br />
General measures<br />
‣ Reduce dose of glucocorticoid when possible,<br />
‣ Consider glucocorticoid sparing therapy if appropriate<br />
or consider alternative route of administration<br />
‣ Recommend good nutrition esp. with adequate calcium<br />
and vit D<br />
‣ Recommend regular weight bearing exercise<br />
‣ Maintain ideal body weight<br />
‣ Avoid tobacco use and alcohol abuse (> government<br />
recommendations)<br />
‣ Assess falls risk and give advice if appropriate<br />
2 of 6 pages
Basingstoke, Southampton and Winchester<br />
District Prescribing Committee<br />
Medical Management of men and women over 45yrs who have<br />
or are at risk of <strong>Osteoporosis</strong><br />
CLINICAL RISK FACTORS FOR OSTEOPOROSIS<br />
Previous fragility fracture*<br />
Predisposing medical<br />
Current glucocorticoid use ≥ 3 months*<br />
conditions<br />
Parental history of hip fracture*<br />
hyperthyroidism<br />
Radiographic osteopenia*<br />
rheumatoid arthritis<br />
Height Loss 3.0 – 5.0 cm*<br />
type 1 diabetes<br />
Female hypogonadism<br />
‣ post-menopause<br />
‣ untreated premature menopause<br />
‣ drug or surgically induced menopause<br />
‣ premenopausal amenorrhoea ≥6 months, (excl pregnancy)<br />
inflammatory bowel<br />
disease<br />
malabsorption/coeliac<br />
disease<br />
prolonged immobility<br />
Body Mass Index ( 3 units alcohol daily<br />
Male hypogonadism ***<br />
Drugs associated with osteoporosis<br />
‣ excessive levothyroxine replacement therapy<br />
‣ long-term heparin<br />
‣ anticonvulsants<br />
‣ antipsychotics<br />
‣ Depo-Provera, 2yrs treatment<br />
‣ Aromatase inhibitors**, GnRH analogues*** (separate guidance)<br />
* Risk factors that clinically indicate a direct Bone Mineral Density assessment.<br />
** Aromatase inhibitor guidance available as algorithms in Appendix 1 to this guideline 1 .<br />
*** The use of GnRH analogues in men is associated with bone loss and fractures but there is no official<br />
guideline to date on its management. Recommend BMD after initiation of therapy (when any additional<br />
secondary causes of bone loss are ruled out) and consider bisphosphonate or denosumab therapy for men<br />
with osteoporosis and fragility fractures, or men with a T score -2.5 SD or lower. Also consider treatment for<br />
men with a T score between -1 and -2.5 SD on GnRH analogues if additional risk factors were present. All<br />
should have adequate intake of calcium and be vitamin D replete or be prescribed supplementation and a have<br />
a follow-up BMD in 1 – 2 years 2 .<br />
The FRAX® tool is an algorithm which calculates fracture risk. (Available at www.shef.ac.uk/FRAX & it links<br />
to guidance published by the National <strong>Osteoporosis</strong> Guideline Group (NOGG) 3 for the management of<br />
osteoporosis).<br />
Therapeutic Agents Available For The Management Of <strong>Osteoporosis</strong><br />
(See Table A for anti-fracture efficacy of therapies available)<br />
Refer to the latest data sheet for full prescribing details about use in elderly, renal and hepatic<br />
impairment, contraindications, precautions etc.<br />
Refer to the BNF- Guidance on prescribing in renal impairment- for advice on using eGFR /<br />
calculated creatinine clearance to adjust doses for patients with renal impairment.<br />
CALCIUM AND VITAMIN D 3<br />
Adequate levels of calcium and vitamin D 3 (colecalciferol) are required to ensure optimum effects of all the<br />
treatments for osteoporosis. Unless the clinician is confident that the patient has adequate calcium intake<br />
and is vitamin D replete, calcium and colecalciferol supplementation at a dose of Calcium 1 – 1.2 gram<br />
(equivalent to 2.5 – 3.0g Calcium Carbonate) and colecalciferol 20 micrograms (800 IU) daily should be<br />
prescribed. Avoid colecalciferol in severe renal impairment as it cannot be converted to its active<br />
form in the renally impaired.<br />
3 of 6 pages
BISPHOSPHONATES<br />
Alendronate is the first choice bisphosphonate for the majority of patients<br />
Risedronate may be prescribed, in patients intolerant of alendronate, in young adults and in<br />
patients with glucocorticoid-induced osteoporosis - may be beneficial due to rapid ‘off’ effect<br />
For other bisphosphonates choices see Table A for site specific anti-fracture efficacy<br />
Intravenous bisphosphonates may be used under specialist guidance<br />
Oral bisphosphonates should be swallowed whole with a glass of water 30-60 minutes before the first food<br />
or drink (other than water) of the day. Patients should stand or sit upright (not lie down) for at least 30<br />
minutes post dose.<br />
Discontinue treatment if oesophageal ulceration, erosion, stricture, or severe lower gastrointestinal<br />
symptoms occur.<br />
Bisphosphonates should be avoided in patients with moderate to severe renal impairment. ( eGFR<br />
< 35ml / minute for alendronate, < 30ml / minute for risedronate).<br />
Atypical femoral fractures (often bilateral) have been reported rarely with bisphosphonate therapy,<br />
mainly in patients receiving long-term treatment for osteoporosis. Patients should be advised to report any<br />
thigh, hip or groin pain. Discontinuation of bisphosphonate therapy in patients suspected to have an<br />
atypical femur fracture should be considered while they are evaluated, and should be based on an<br />
assessment of the benefits and risks of treatment. For general advice on duration of therapy see separate<br />
section ‘Duration of Treatment’ on page 5 of the <strong>guidelines</strong>.<br />
Osteonecrosis of the jaw has been reported rarely with IV bisphosphonate use and very rarely with oral<br />
use. Adequate oral hygiene should be maintained during and after bisphosphonate treatment. Ideally in<br />
patients with concomitant risk factors e.g. cancer, chemotherapy treatment, glucocorticoid treatment, or<br />
poor oral hygiene, remedial dental work should be completed before starting bisphosphonates.<br />
STRONTIUM RANELATE<br />
Indicated, when bisphosphonates are contra-indicated or cannot be tolerated, or the patient has difficulty<br />
complying with their strict ingestion regimen.<br />
Also consider for<br />
<br />
women aged over 80 years for the primary prevention of fragility fractures<br />
women aged 75 years or older with a previous fragility fracture<br />
Strontium should be taken at bedtime at least 2 hours after food and /or milk.<br />
Avoid in severe renal impairment ( eGFR 30ml/min).<br />
Severe allergic reactions including DRESS (drug rash with eosinophilia systemic symptoms) have been<br />
reported in patients taking strontium. If a rash develops, treatment must be stopped immediately. The risk<br />
of severe allergic reactions can be life-threatening and strontium must not be re-introduced. Symptoms can<br />
be improved by glucorticoid therapy but recovery can be slow and there is a risk of symptoms returning<br />
during the recovery period.<br />
Risk of thrombo-embolism is increased so assess predisposing conditions prior to use<br />
RALOXIFENE<br />
For postmenopausal women with vertebral osteoporosis, with an unsatisfactory response to or an<br />
intolerance of bisphosphonates. Avoid in severe renal impairment.<br />
TERIPARATIDE (Specialist Use only)<br />
Indications restricted to patients with an unsatisfactory response/intolerance to the above therapies AND<br />
<br />
<br />
<br />
aged > 65 yrs old who have a T score of –4 SD or below OR<br />
aged > 65 yrs old who have a T score of -3.5 SD or below plus at least 2 fractures OR<br />
aged 55 – 64 yrs old who have a T score of -4 SD or below plus at least 2 fractures<br />
Use with caution in moderate renal impairment. Contraindicated in severe renal impairment.<br />
4 of 6 pages
DENOSUMAB (Specialist Initiated AMBER drug)<br />
(Shared Care Guidelines at www.hampshire.nhs.uk/primary-care on Pages4PrimaryCare website, Prescribing Folder in GP & Pharmacist<br />
sections).<br />
<br />
For the secondary prevention of osteoporotic fragility fractures in postmenopausal women at<br />
increased risk of fractures who are unable to comply with the special instructions for the<br />
administration of oral bisphosphonates, are intolerant of oral bisphosphonates or for whom<br />
treatment with oral bisphosphonates is contraindicated.<br />
<br />
<br />
<br />
Administered as a 60mg subcutaneous injection at 6 month intervals<br />
No dose adjustment required in patients with renal impairment.<br />
Ensure calcium levels are within the normal range prior to initiation of, and during therapy.<br />
N.B. To decrease the possibility of duplication of bone protection prescribing it is essential<br />
that;<br />
a) secondary care inform the patient’s GP of the date denosumab therapy was initiated<br />
and<br />
b) primary care are advised that these details are included in the patient’s repeat medication records<br />
and entered onto practice recall system for recall at 6 month intervals.<br />
The following conditions may be associated with denosumab treatment: eczema, diverticulitis, cataracts,<br />
hypocalcaemia, and skin infections (predominantly cellulitis). The SPC states that osteonecrosis of the jaw<br />
has been reported in patients receiving denosumab or bisphosphonates with most cases occurring in<br />
cancer patients; however some have occurred in patients with osteoporosis.<br />
HORMONE REPLACEMENT THERAPY<br />
<br />
only recommended as treatment for the prevention of osteoporosis in women with a premature<br />
menopause, and only prescribed in women up to 50 years of age.<br />
COMBINATION THERAPY<br />
(not including combinations with Calcium and colecalciferol)<br />
<br />
is not routinely prescribed but may be rarely used under specialist recommendation only.<br />
DURATION OF TREATMENT<br />
Oral bisphosphonates, strontium ranelate and raloxifene are recommended for up to five years of<br />
treatment followed by re-evaluation of the individual patient. DXA scan or evaluation of biochemical<br />
markers of bone turnover can be considered after this period. After five years of treatment, for patients not<br />
considered at high risk of fracture, a “drug holiday period” of up to three years without therapy can be<br />
considered. (N.B Ensure patient is calcium and vitamin D replete, or continue adequate supplementation).<br />
In other circumstances, it appears safe to continue for a further five years of treatment. Teriparatide should<br />
be used for up to 18 months, and denosumab for up to 3 years in the first instance.<br />
TABLE A 3<br />
Effect of major pharmacological interventions on fracture risk when<br />
given with calcium and vitamin D in postmenopausal women with osteoporosis<br />
Intervention Vertebral Non-vertebral Hip<br />
Alendronate A A A<br />
Risedronate A A A<br />
Zoledronate A A A<br />
Denosumab A A A<br />
Strontium ranelate A A A<br />
Ibandronate a A A nae<br />
Raloxifene A nae nae<br />
PTH (1-84) A nae nae<br />
Teriparatide A A nae<br />
in subsets of patients only (post-hoc analysis)<br />
a<br />
Injection only available on Formulary<br />
nae : not adequately evaluated<br />
PTH : recombinant human parathyroid hormone<br />
5 of 6 pages
Table A 3<br />
Grading of recommendations and evidence levels<br />
Levels of evidence for studies of intervention are defined as follows:<br />
Ia from meta-analysis of randomised controlled trials (RCTs)<br />
Ib from at least one RCT<br />
IIa from at least one well designed controlled study without randomisation<br />
IIb from at least one other type of well designed quasi-experimental study<br />
III from well designed non-experimental descriptive studies, eg comparative<br />
studies, correlation studies, case-control studies<br />
IV from expert committee reports or opinions and/or clinical experience of<br />
authorities<br />
The validity of candidate risk factors is also assessed by an evidence-based approach:<br />
Ia Systematic reviews or meta-analysis of level I studies with a high degree of homogeneity<br />
Ib Systematic reviews or meta-analysis with moderate or poor homogeneity<br />
Ic Level I studies (with appropriate populations and internal controls)<br />
IIa Systematic reviews or meta-analysis of level II studies<br />
IIb Level II studies (inappropriate population or lacking an internal control)<br />
IIIa Systematic reviews or meta-analysis of level III studies<br />
IIIb Case-control studies<br />
IV Evidence from expert committees without explicit critical scientific analysis or that based on<br />
physiology, basic research or first principles.<br />
The quality of the guideline recommendations is similarly graded to indicate the levels of<br />
evidence on which they are based:<br />
grade A evidence levels Ia and Ib<br />
grade B evidence levels IIa, IIb and III<br />
grade C evidence level IV<br />
References<br />
1. Reid DM, Doughty J, Eastell R et al. Guidance for the management of breast cancer treatment-induced<br />
bone loss: a consensus position statement from a UK Expert Group. 2008. Cancer Treat Rev 2008; 34:<br />
S1– S18.<br />
(Full guidance and 2 treatment algorithms available at www.nos.org.uk/NetCommunity/Document.Doc?id=124<br />
and the algorithms are also contained in Appendix 1 of this document.<br />
2. Greenspan S. Approach to the Prostate Cancer Patient with Bone Disease. J Clin Endocrinol Metab.<br />
Jan 2008, 93(1):2-7<br />
3. Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the<br />
age of 50 years in the UK. Produced by J Compston, A Cooper, C Cooper, R Francis, JA Kanis, D Marsh,<br />
EV McCloskey, DM Reid, P Selby and M Wilkins, on behalf of the National <strong>Osteoporosis</strong> Guideline Group<br />
(NOGG). October 2008 and updated <strong>July</strong> 2010 (www.shef.ac.uk/NOGG/ )<br />
4. Bone and Tooth Society of Great Britain, National <strong>Osteoporosis</strong> Society, Royal College of Physicians<br />
“Glucocorticoid – induced <strong>Osteoporosis</strong>: Guidelines for Prevention and Treatment” London: RCP, 2002<br />
5. Bone and Tooth Society of Great Britain, National <strong>Osteoporosis</strong> Society, Royal College of Physicians<br />
‘‘Clinical Guidelines for the prevention and treatment of <strong>Osteoporosis</strong>’’ London RCP Update 2000<br />
6. Reginster JY, Seeman E, De Vernejoul MC et al. Strontium ranelate reduces the risk of nonvertebral<br />
fractures in postmenopausal women with osteoporosis: Treatment of Peripheral <strong>Osteoporosis</strong> (TROPOS)<br />
study. J Clin Endocrinol Metab.2005 May; 90(5):2816-22.<br />
7. Meunier PJ, Roux C, Seeman E, et al. The effects of strontium ranelate on the risk of vertebral fracture in<br />
women with postmenopausal osteoporosis. N Engl J Med. 2004 Jan 29; 350(5):459-68.<br />
8. NICE Technology Appraisal TA161 – Alendronate, etidronate, risedronate, raloxifene, strontium ranelate<br />
and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women.<br />
October 2008.<br />
9. NICE Technology Appraisal TA160- Alendronate, etidronate, risedronate, raloxifene and strontium ranelate<br />
for the primary prevention of osteoporotic fragility fractures in post menopausal women. October 2008.<br />
10. NICE Technology Apprasal TA 204 -Denosumab for the prevention of osteoporotic fractures in<br />
postmenopausal women. October 2010.<br />
Prepared by:<br />
Approved by:<br />
Date:<br />
Kathleen Hayes, Pharmacist ,Falls Team, Solent <strong>NHS</strong> Trust, in collaboration with Prof. C Cooper,<br />
Professor of Rheumatology, Dr E Dennison, Consultant Rheumatologist, University of<br />
Southampton School of Medicine, Dr Gill Pearson, Associate Specialist in Rheumatology,<br />
Southampton, Dr Annie Cooper, Consultant Rheumatologist, Winchester, Dr Peter Prouse,<br />
Consultant Rheumatologist, Basingstoke.<br />
Basingstoke Southampton and Winchester District Prescribing Committee<br />
<strong>July</strong> <strong>2011</strong> Renewal date <strong>July</strong> 2012<br />
6 of 6 pages
Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, Powles T, Selby P, Coleman RE. Guidance for the management of breast cancer treatmentinduced<br />
bone loss: a consensus position statement from a UK Expert Group. 2008. Cancer Treat Rev 2008;34:S1–S18.<br />
Appendix 1 Management of bone loss in early breast cancer