Low-dose of thalidomide in the treatment of refractory myeloma
Low-dose of thalidomide in the treatment of refractory myeloma
Low-dose of thalidomide in the treatment of refractory myeloma
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
scientific correspondence<br />
1111<br />
reported 5 cases <strong>of</strong> PTCL, all with a long-last<strong>in</strong>g<br />
history <strong>of</strong> granulomatosis, hepatosplenomegaly,<br />
HBsAg and EBV-VCA positivity. The only surviv<strong>in</strong>g<br />
patient had received an allogeneic bone-marrow<br />
transplantation. Our two children had been<br />
<strong>in</strong>fected by EBV with a chronic high titer <strong>of</strong> IgG<br />
aga<strong>in</strong>st EBV-VCA, consistent with a life-long<br />
<strong>in</strong>fection. 9 EBV may <strong>in</strong>fect T-cells creat<strong>in</strong>g reactive<br />
lymphoid proliferations without contribut<strong>in</strong>g<br />
to <strong>the</strong> neoplastic process; however EBV or o<strong>the</strong>r<br />
viral <strong>in</strong>fections (i.e. Cytomegalovirus, hepatitis<br />
B), or immune defects suppress<strong>in</strong>g NK activity –<br />
such as altered T4/T8 ratio – may lead to neoplastic<br />
transformation <strong>in</strong> particular hosts, and<br />
this could be <strong>the</strong> case with our patients. All available<br />
lymph node samples were <strong>the</strong>refore submitted<br />
to EBV-DNA detection without f<strong>in</strong>d<strong>in</strong>g<br />
amplification <strong>of</strong> <strong>the</strong> viral genome. The presence<br />
<strong>of</strong> EBV-DNA is a feature <strong>of</strong> angioimmunoblastic<br />
PTCL found <strong>in</strong> Eastern Countries, be<strong>in</strong>g less<br />
common <strong>in</strong> Europe. 3,10 As to optimal <strong>treatment</strong>,<br />
we agree with <strong>the</strong> Taiwan group: 3 marrow transplantation<br />
can cure this disease. The role <strong>of</strong><br />
ret<strong>in</strong>oic acid cannot be assessed by anecdotal<br />
experiences, even though malignant cell differentiation<br />
and apoptosis might depend on<br />
ret<strong>in</strong>oic acid adm<strong>in</strong>istration. 10 No national or<br />
<strong>in</strong>ternational co-operative group is currently<br />
deal<strong>in</strong>g with poor-prognosis PTCL: a common<br />
study is <strong>the</strong>refore needed, perhaps <strong>in</strong>clud<strong>in</strong>g<br />
both adults and children.<br />
Maura Massim<strong>in</strong>o, Daniela Perotti, Filippo Spreafico,<br />
Roberto Luksch, Alberto Garaventa,° Roberto Giard<strong>in</strong>i<br />
Istituto Nazionale Tumori, Milan; °Istituto G. Gasl<strong>in</strong>i, Genoa, Italy<br />
Fund<strong>in</strong>g<br />
This work was partially supported by Associazione<br />
Bianca Garavaglia, Busto Arsizio (VA) and AIRC.<br />
Key words<br />
Childhood non-Hodgk<strong>in</strong>’s lymphoma, PTCL, ALCL.<br />
Correspondence<br />
Maura Massim<strong>in</strong>o, M.D., Division <strong>of</strong> Pediatric<br />
Oncology, Istituto Nazionale Tumori, via G. Venezian,<br />
1, 20133 Milan, Italy. Phone: <strong>in</strong>ternational +39-02-<br />
2390593 – Fax: <strong>in</strong>ternational +39-02-2665642 –<br />
E-mail: massim<strong>in</strong>o@istitutotumori.mi.it<br />
References<br />
1. Harris NL, Jaffe ES, Ste<strong>in</strong> H, et al. A revised European-<br />
American classification <strong>of</strong> lymphoid neoplasms: a proposal<br />
from <strong>the</strong> International Lymphoma Study Group.<br />
Blood 1994; 84:1361-92<br />
2. Jaffe ES, Harris NL, Diebold J, et al. World Health<br />
Organization classification <strong>of</strong> neoplastic diseases <strong>of</strong><br />
<strong>the</strong> hematopoietic and lymphoid tissues. A progress<br />
report. Am J Cl<strong>in</strong> Pathol 1999, 111, S8-12.<br />
3. L<strong>in</strong> KH, Su IJ, Chen RL, et al. Peripheral T-cell lymphoma<br />
<strong>in</strong> childhood: a report <strong>of</strong> five cases <strong>in</strong> Taiwan.<br />
Med Pediatr Oncol 1994; 23:26-32.<br />
4. Agnarsson BA, Kad<strong>in</strong> ME. Peripheral T-cell lymphomas<br />
<strong>in</strong> children. Sem Diagn Pathol 1995; 12:314-<br />
24.<br />
5. Neuhauser TS, Lancaster K, Haws R, et al. Rapidly<br />
progressive T cell lymphoma present<strong>in</strong>g as acute renal<br />
failure: case report and review <strong>of</strong> <strong>the</strong> literature. Pediatr<br />
Pathol Lab Med 1997; 17:449-60.<br />
6. de Terlizzi M, Toma MG, Santostasi T, et al. Angioimmunoblastic<br />
lymphadenopathy with dysprote<strong>in</strong>emia:<br />
report <strong>of</strong> a case <strong>in</strong> <strong>in</strong>fancy with review <strong>of</strong> literature.<br />
Pediatr Hematol Oncol 1989; 6:37-44.<br />
7. Leake J, Kellie SJ, Pritchard J, et al. Peripheral T-cell<br />
lymphoma <strong>in</strong> childhood. A cl<strong>in</strong>icopathologic study <strong>of</strong><br />
six cases. Histopathology 1989; 14:225-8.<br />
8. Cheng AL, Su IJ, Chen CC, et al. Characteristic cl<strong>in</strong>ico-pathologic<br />
feature <strong>of</strong> Epste<strong>in</strong>-Barr virus associated<br />
peripheral T-cell lymphoma. Cancer 1993; 72:909-16.<br />
9. Jones JF, Shur<strong>in</strong> S, Abramowsky C, et al. T-cell lymphomas<br />
conta<strong>in</strong><strong>in</strong>g Epste<strong>in</strong>-Barr viral DNA <strong>in</strong> patients<br />
with chronic Epste<strong>in</strong>-Barr virus <strong>in</strong>fections. N Engl J<br />
Med 1988; 318:733-41.<br />
10. Cheng AL, Su IJ, Chen CC, et al. Use <strong>of</strong> ret<strong>in</strong>oic acids<br />
<strong>in</strong> <strong>the</strong> <strong>treatment</strong> <strong>of</strong> peripheral T-cell lymphoma: a pilot<br />
study. J Cl<strong>in</strong> Oncol 1994; 12:1185-92.<br />
<strong>Low</strong>-<strong>dose</strong> <strong>thalidomide</strong> <strong>in</strong> <strong>the</strong> <strong>treatment</strong> <strong>of</strong><br />
<strong>refractory</strong> <strong>myeloma</strong><br />
In this report we present five cases <strong>of</strong> <strong>refractory</strong><br />
multiple <strong>myeloma</strong> successfully treated<br />
with low <strong>dose</strong>s <strong>of</strong> <strong>thalidomide</strong>.<br />
Sir,<br />
Barlogie’s group recently reported <strong>the</strong> results<br />
<strong>of</strong> a phase 2 study with <strong>thalidomide</strong>, as a s<strong>in</strong>gle<br />
agent, <strong>in</strong> <strong>the</strong> <strong>treatment</strong> <strong>of</strong> <strong>refractory</strong> <strong>myeloma</strong>. 1<br />
In this study <strong>the</strong> rate <strong>of</strong> response was 32%, as<br />
shown by a reduction <strong>of</strong> at least 25% <strong>of</strong> <strong>the</strong> M-<br />
component. The study considered a gradual<br />
<strong>in</strong>crease <strong>in</strong> <strong>the</strong> <strong>dose</strong> <strong>of</strong> <strong>thalidomide</strong> up to a maximum<br />
daily <strong>dose</strong> <strong>of</strong> 800 mg. The toxicity l<strong>in</strong>ked<br />
to <strong>the</strong> <strong>treatment</strong> was not negligible. Fur<strong>the</strong>rmore,<br />
<strong>in</strong> <strong>the</strong> last six months several prelim<strong>in</strong>ary<br />
reports have shown <strong>the</strong> efficacy <strong>of</strong> low <strong>dose</strong><br />
<strong>thalidomide</strong> <strong>in</strong> <strong>the</strong> <strong>treatment</strong> <strong>of</strong> resistant <strong>myeloma</strong>.<br />
2-8 We present our experience on a small<br />
group <strong>of</strong> patients with <strong>refractory</strong> <strong>myeloma</strong> treated<br />
with low <strong>dose</strong> <strong>thalidomide</strong>. The study <strong>in</strong>cluded<br />
five patients (4 males, 1 female; median age<br />
67 years, range 58-76). All patients had received<br />
two or more different regimens <strong>of</strong> conventional<br />
<strong>the</strong>rapy. No patient had been submitted to high<br />
<strong>dose</strong> chemo<strong>the</strong>rapy with autologous hematopoietic<br />
stem cell transplantation. At <strong>the</strong> start <strong>of</strong><br />
<strong>thalidomide</strong> <strong>the</strong>rapy all patients had progressive<br />
disease. The patients started with 200 mg/day<br />
<strong>of</strong> <strong>thalidomide</strong> as a s<strong>in</strong>gle <strong>the</strong>rapeutic agent<br />
after provid<strong>in</strong>g written consent. The <strong>dose</strong> has<br />
not been <strong>in</strong>creased dur<strong>in</strong>g <strong>the</strong> whole period <strong>of</strong><br />
<strong>the</strong> <strong>treatment</strong>. In our patients <strong>the</strong> side effects<br />
were mild or moderate (grade 1 or 2 accord<strong>in</strong>g<br />
Haematologica vol. 85(10):October 2000
1112<br />
scientific correspondence<br />
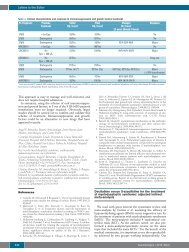
Table 1.<br />
Patient #1 Patient #2 Patient #3<br />
S maxR % S maxR % S maxR %<br />
Paraprote<strong>in</strong> levels (g/dL)<br />
6 0.8 87 3 0.3 90 3.3 1.6 51.5<br />
Bone marrow plasmacytosis (%)<br />
60 11 82 82 30 63 30 10 66<br />
β2-microglobul<strong>in</strong> (mg/L)<br />
10.4 3.2 69 8 4.9 39 3.82 1.73 55<br />
S= start<strong>in</strong>g value; MaxR= <strong>the</strong> best response; %= percentage <strong>of</strong> response.<br />
to WHO criteria). The <strong>dose</strong> <strong>of</strong> <strong>thalidomide</strong> was<br />
reduced to 100 mg/day <strong>in</strong> 2 patients because <strong>of</strong><br />
constipation and a transient reduction <strong>of</strong><br />
platelets. The median time <strong>of</strong> <strong>treatment</strong> has<br />
been 5 months (range 2-9). Four <strong>of</strong> five patients<br />
(80%) have responded to <strong>the</strong> <strong>the</strong>rapy – two<br />
patients with more than 70% reduction <strong>of</strong> <strong>the</strong><br />
M-component levels, one with more than 50%<br />
and <strong>the</strong> last one with about a 30% reduction.<br />
The fifth patient had no response and stopped<br />
<strong>the</strong> <strong>the</strong>rapy after two months. The <strong>in</strong>terval <strong>of</strong><br />
time necessary to obta<strong>in</strong> <strong>the</strong> best rate <strong>of</strong><br />
response to <strong>the</strong> <strong>the</strong>rapy was 50 days (range 34-<br />
64). In <strong>the</strong> three patients with <strong>the</strong> best response<br />
to <strong>the</strong> <strong>the</strong>rapy a reduction <strong>in</strong> <strong>the</strong> percentage <strong>of</strong><br />
plasma cells <strong>in</strong> bone marrow, β2 microglobul<strong>in</strong><br />
levels and C reactive prote<strong>in</strong> was evident (Table<br />
1). Fur<strong>the</strong>rmore, a good response was obta<strong>in</strong>ed<br />
<strong>in</strong> one patient who reduced <strong>the</strong> <strong>dose</strong> <strong>of</strong><br />
<strong>thalidomide</strong> to 100 mg/die early <strong>in</strong> <strong>treatment</strong>. In<br />
conclusion, although our experience concerns<br />
only five cases, we confirm <strong>the</strong> results published<br />
<strong>in</strong> scientific literature 1-8 on <strong>the</strong> efficacy <strong>of</strong><br />
<strong>thalidomide</strong> <strong>in</strong> <strong>myeloma</strong> <strong>the</strong>rapy. We po<strong>in</strong>t out<br />
<strong>the</strong> possibility <strong>of</strong> obta<strong>in</strong><strong>in</strong>g a good objective<br />
response 9 with lower and better tolerated <strong>dose</strong><br />
<strong>of</strong> drug. We, <strong>the</strong>refore, th<strong>in</strong>k it would be desirable<br />
to study <strong>the</strong> most suitable <strong>dose</strong> <strong>of</strong> <strong>thalidomide</strong><br />
<strong>in</strong> a larger number <strong>of</strong> patients.<br />
Massimo P<strong>in</strong>i, Anna Baraldi, Daniela Pietrasanta,<br />
Bernard<strong>in</strong>o Allione, Lorella Depaoli, Flavia Salvi, Alessandro Levis<br />
Hematology Division, Azienda Ospedaliera SS. Antonio e Biagio,<br />
Alessandria, Italy<br />
Key words<br />
Multiple <strong>myeloma</strong>, low <strong>dose</strong> <strong>thalidomide</strong>, side effects.<br />
Correspondence<br />
Massimo P<strong>in</strong>i M.D., Hematology Division, Az.<br />
Ospedaliera SS. Antonio e Biagio C. Arrigo, via Venezia<br />
16, 15100 Alessandria, Italy. Phone: <strong>in</strong>ternational<br />
+39-0131-206809 – Fax: <strong>in</strong>ternational +39-0131-<br />
261029 – E-mail: alevis@ospedale.al.it<br />
References<br />
1. S<strong>in</strong>ghal S, Metha J, Desikan R, et al. Antitumor activity<br />
<strong>of</strong> <strong>thalidomide</strong> <strong>in</strong> <strong>refractory</strong> multiple <strong>myeloma</strong>. N<br />
Engl J Med 1999; 341:1565-71.<br />
2. Barlogie B, Desikan R, Munshi N, et al. S<strong>in</strong>gle course<br />
D.T. PACE antiangiochemo<strong>the</strong>rapy effects CR <strong>in</strong> plasma<br />
cells leukemia and fulm<strong>in</strong>ant multiple <strong>myeloma</strong><br />
(MM). Blood 1998; Suppl 1: 273b.<br />
3. Kneller A, Raanani P, Hardan I, et al. Therapy with<br />
<strong>thalidomide</strong> <strong>in</strong> <strong>refractory</strong> multiple <strong>myeloma</strong> patients –<br />
<strong>the</strong> revival <strong>of</strong> an old drug. Br J Haematol 2000; 108:<br />
391-3.<br />
4. Durie BGM, Stephan DE. Efficacy <strong>of</strong> low <strong>dose</strong> <strong>thalidomide</strong><br />
(T) <strong>in</strong> multiple <strong>myeloma</strong>. Blood 1999; Suppl<br />
1413.<br />
5. Chen CI, Adesanya A, Sutton DM, et al. <strong>Low</strong>-<strong>dose</strong><br />
Thalidomide <strong>in</strong> patients with advanced, <strong>refractory</strong><br />
multiple <strong>myeloma</strong>. Blood 1999; Suppl: 4603 abstract.<br />
6. Sabir T, Raza S, Anderson L, et al. Thalidomide is<br />
effective <strong>in</strong> <strong>the</strong> <strong>treatment</strong> <strong>of</strong> recurrent, <strong>refractory</strong> multiple<br />
<strong>myeloma</strong> (MM). Blood 1999; Suppl 548.<br />
7. Zomas A, Anagnostopoulos N, Dimopoulos MA. Successful<br />
<strong>treatment</strong> <strong>of</strong> multiple <strong>myeloma</strong> relaps<strong>in</strong>g after<br />
high-<strong>dose</strong> <strong>the</strong>rapy and autologous transplantation<br />
with <strong>thalidomide</strong> as s<strong>in</strong>gle agent. Bone Marrow Transplant<br />
2000; 25:1319-20.<br />
8. Juliusson G, Cels<strong>in</strong>g F, Turesson I, Lenh<strong>of</strong>f S, Adriansson<br />
M, Malm C. Frequent good partial remissions<br />
from <strong>thalidomide</strong> <strong>in</strong>clud<strong>in</strong>g best response ever <strong>in</strong><br />
patients with advanced <strong>refractory</strong> and relapsed <strong>myeloma</strong>.<br />
Br J Haematol 2000; 109:89-96.<br />
9. San Miguel JF, Blade Creixenti J, Garcia-Sanz R. Treatment<br />
<strong>of</strong> multiple <strong>myeloma</strong>. Haematologica 1999; 84:<br />
36-58.<br />
Feasibility and efficacy <strong>of</strong> high-<strong>dose</strong> etoposide<br />
followed by low-<strong>dose</strong> granulocyte colony-stimulat<strong>in</strong>g<br />
factor as a mobilization regimen <strong>in</strong><br />
patients with non-Hodgk<strong>in</strong>’s lymphoma<br />
The adm<strong>in</strong>istration <strong>of</strong> high-<strong>dose</strong> (HD)<br />
etoposide (ETP) with higher <strong>dose</strong>s <strong>of</strong> recomb<strong>in</strong>ant<br />
human (rh) granulocyte colony-stimulat<strong>in</strong>g<br />
factor (G-CSF) is useful for peripheral<br />
blood stem cell (PBSC) mobilization. 1-3 We<br />
report <strong>the</strong> efficacy <strong>of</strong> HD-ETP with low-<strong>dose</strong><br />
rhG-CSF for PBSC mobilization <strong>in</strong> patients<br />
with non-Hodgk<strong>in</strong>’s lymphoma (NHL).<br />
Sir,<br />
Twenty-three newly diagnosed patients (16<br />
men and 7 women) with NHL were <strong>in</strong>cluded <strong>in</strong><br />
this study.Their median age was 51 years (range,<br />
20-66). They were treated with 2 or 3 courses <strong>of</strong><br />
CHOP <strong>the</strong>rapy as an <strong>in</strong>duction <strong>the</strong>rapy. Twelve<br />
patients entered complete remission (CR), 10<br />
patients partial remission (PR), and one patient<br />
had no change. Patients were treated with ETP<br />
500 mg/m 2 i.v. for 2 hour daily on days 1 to 3.<br />
rhG-CSF (Kir<strong>in</strong> Brewery Co., Tokyo, Japan) was<br />
adm<strong>in</strong>istered by s.c. <strong>in</strong>jection at a <strong>dose</strong> <strong>of</strong> 50<br />
µg/m 2 on <strong>the</strong> second day after completion <strong>of</strong><br />
Haematologica vol. 85(10):October 2000