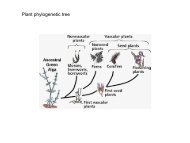
Edwards et al., Curr Opin Struct Biol 2007
Edwards et al., Curr Opin Struct Biol 2007
Edwards et al., Curr Opin Struct Biol 2007
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
M<strong>et</strong>abolite recognition by riboswitches <strong>Edwards</strong>, Klein and Ferré-D’Amaré 279<br />
16. Klein DJ, Schmeing TM, Moore PB, Steitz TA: The kink-turn:<br />
a new RNA secondary structure motif. EMBO J 2001,<br />
20:4214-4221.<br />
17. Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR: Control of<br />
gene expression by a natur<strong>al</strong> m<strong>et</strong>abolite-responsive ribozyme.<br />
Nature 2004, 428:281-286.<br />
18.<br />
<br />
Klein DJ, Ferré-D’Amaré AR: <strong>Struct</strong>ur<strong>al</strong> basis of glmS ribozyme<br />
activation by glucosamine-6-phosphate. Science 2006,<br />
313:1752-1756.<br />
Cryst<strong>al</strong> structures of the glmS ribozyme in pre- and post-cleavage states,<br />
tog<strong>et</strong>her with the demonstration of ribozyme activity in the cryst<strong>al</strong>line<br />
state, indicate that this riboswitch is remarkably rigid and folds in the<br />
absence of effector. The structure of the ribozyme bound to the antagonist<br />
Glc6P suggests how the activator functions as a coenzyme.<br />
19.<br />
<br />
Cochrane JC, Lipchock SV, Strobel SA: <strong>Struct</strong>ur<strong>al</strong> investigation<br />
of the glmS ribozyme bound to its cat<strong>al</strong>ytic cofactor.<br />
Chem <strong>Biol</strong> <strong>2007</strong>, 14:97-105.<br />
The structure of the glmS ribozyme bound to the activator GlcN6P<br />
confirms that its mode of binding is similar to that of the antagonist<br />
Glc6P. Kin<strong>et</strong>ic an<strong>al</strong>yses show that the K m but not the k cat of the reaction is<br />
pH dependent.<br />
20.<br />
<br />
Hampel KJ, Tinsley MM: Evidence for preorganization of the<br />
glmS ribozyme ligand binding pock<strong>et</strong>. Biochemistry 2006,<br />
45:7861-7871.<br />
Biochemic<strong>al</strong> an<strong>al</strong>ysis of the glmS ribozyme-riboswitch provides evidence<br />
that the activator-binding site is largely pre-organized.<br />
21. Rupert PB, Ferré-D’Amaré AR: Cryst<strong>al</strong> structure of a hairpin<br />
ribozyme-inhibitor complex with implications for cat<strong>al</strong>ysis.<br />
Nature 2001, 410:780-786.<br />
22. Rupert PB, Massey AP, Sigurdsson ST, Ferré-D’Amaré AR:<br />
Transition state stabilization by a cat<strong>al</strong>ytic RNA. Science 2002,<br />
298:1421-1424.<br />
23. Serganov A, Keiper S, M<strong>al</strong>inina L, Tereshko V, Skripkin E,<br />
Hobartner C, Polonskaia A, Phan AT, Wombacher R, Micura R<br />
<strong>et</strong> <strong>al</strong>.: <strong>Struct</strong>ur<strong>al</strong> basis for Diels-Alder ribozyme-cat<strong>al</strong>yzed<br />
carbon-carbon bond formation. Nat <strong>Struct</strong> Mol <strong>Biol</strong> 2005,<br />
12:218-224.<br />
24.<br />
<br />
Gilbert SD, Stoddard CD, Wise SJ, Batey RT: Thermodynamic<br />
and kin<strong>et</strong>ic characterization of ligand binding to the purine<br />
riboswitch aptamer domain. J Mol <strong>Biol</strong> 2006, 359:754-768.<br />
The authors present a biophysic<strong>al</strong> and structur<strong>al</strong> an<strong>al</strong>ysis of the specificity<br />
of the purine riboswitch, in particular, the importance of Watson–Crick<br />
pairing of the effector with pyrimidine 74.<br />
25. Gilbert SD, Mediatore SJ, Batey RT: Modified pyrimidines<br />
specific<strong>al</strong>ly bind the purine riboswitch. J Am Chem Soc 2006,<br />
128:14214-14215.<br />
26. Noeske J, Richter C, Grundl MA, Nasiri HR, Schw<strong>al</strong>be H,<br />
Wöhnert J: An intermolecular base triple as the basis of ligand<br />
specificity and affinity in the guanine- and adenine-sensing<br />
riboswitch RNAs. Proc Natl Acad Sci USA 2005, 102:1372-1377.<br />
27. Dieckmann T, Suzuki E, Nakamura GK, Feigon J: Solution<br />
structure of an ATP-binding RNA aptamer reve<strong>al</strong>s a novel fold.<br />
RNA 1996, 2:628-640.<br />
28. Jiang F, Kumar RA, Jones RA, Patel DJ: <strong>Struct</strong>ur<strong>al</strong> basis of RNA<br />
folding and recognition in an AMP-RNA aptamer complex.<br />
Nature 1996, 382:183-186.<br />
29. Noeske J, Richter C, Stirn<strong>al</strong> E, Schw<strong>al</strong>be H, Wöhnert J:<br />
Phosphate-group recognition by the aptamer domain of the<br />
thiamine pyrophosphate sensing riboswitch. ChemBioChem<br />
2006, 7:1451-1456.<br />
30. Lim J, Winkler WC, Nakamura S, Scott V, Breaker RR: Molecularrecognition<br />
characteristics of SAM-binding riboswitches.<br />
Angew Chem Int Ed Engl 2006, 45:964-968.<br />
31. Auffinger P, Bielecki L, Westhof E: Anion binding to nucleic<br />
acids. <strong>Struct</strong>ure 2004, 12:379-388.<br />
32. Wilson DS, Szostak JW: In vitro selection of function<strong>al</strong> nucleic<br />
acids. Annu Rev Biochem 1999, 68:611-647.<br />
33. Hermann T, Patel DJ: Adaptive recognition by nucleic acid<br />
aptamers. Science 2000, 287:820-825.<br />
34. Ferré-D’Amaré AR, Doudna JA: RNA folds: insights from<br />
recent cryst<strong>al</strong> structures. Annu Rev Biophys Biomol <strong>Struct</strong> 1999,<br />
28:57-73.<br />
35. Eisenberg D, McLachlan AD: Solvation energy in protein folding<br />
and binding. Nature 1986, 319:199-203.<br />
36. Nahvi A, Sudarsan N, Ebert MS, Zou X, Brown KL, Breaker RR:<br />
Gen<strong>et</strong>ic control by a m<strong>et</strong>abolite binding mRNA. Chem <strong>Biol</strong><br />
2002, 9:1043.<br />
37. Müller M, Weigand JE, Weichenrieder O, Suess B:<br />
Thermodynamic characterization of an engineered<br />
t<strong>et</strong>racycline-binding riboswitch. Nucleic Acids Res 2006,<br />
34:2607-2617.<br />
38. Mand<strong>al</strong> M, Lee M, Barrick JE, Weinberg Z, Emilsson GM,<br />
Ruzzo WL, Breaker RR: A glycine-dependent riboswitch<br />
that uses cooperative binding to control gene expression.<br />
Science 2004, 306:275-279.<br />
39. Carothers JM, Oestreich SC, Davis JH, Szostak JW:<br />
Information<strong>al</strong> complexity and function<strong>al</strong> activity of RNA<br />
structures. J Am Chem Soc 2004, 126:5130-5137.<br />
40.<br />
<br />
Carothers JM, Oestreich SC, Szostak JW: Aptamers selected for<br />
higher-affinity binding are not more specific for the targ<strong>et</strong><br />
ligand. J Am Chem Soc 2006, 128:7929-7937.<br />
The authors an<strong>al</strong>yzed the effect of structur<strong>al</strong> complexity on affinity and<br />
selectivity for 11 distinct classes of GTP aptamers.<br />
41. Lemay JF, Penedo JC, Tremblay R, Lilley DM, Lafontaine DA:<br />
Folding of the adenine riboswitch. Chem <strong>Biol</strong> 2006, 13:857-868.<br />
Ensemble and single-molecule FRET measurements demonstrated multiple<br />
Mg 2+ -dependent folded states of the adenine riboswitch in the<br />
absence of ligand.<br />
42.<br />
<br />
Noeske J, Buck J, Furtig B, Nasiri HR, Schw<strong>al</strong>be H, Wöhnert J:<br />
Interplay of ’induced fit’ and preorganization in the ligand<br />
induced folding of the aptamer domain of the guanine binding<br />
riboswitch. Nucleic Acids Res <strong>2007</strong>, 35:101-112.<br />
Solution NMR an<strong>al</strong>ysis of a guanine riboswitch reve<strong>al</strong>s pre-organization of<br />
the glob<strong>al</strong> fold, including the termin<strong>al</strong> loop–loop interaction, in the<br />
absence of effector.<br />
43. Lipfert J, Das R, Chu VB, Kudarav<strong>al</strong>li M, Boyd N, Herschlag D,<br />
Doniach S: <strong>Struct</strong>ur<strong>al</strong> transitions and thermodynamics of a<br />
glycine-dependent riboswitch from Vibrio cholerae.<br />
J Mol <strong>Biol</strong> <strong>2007</strong>, 365:1393-1406.<br />
44. Mironov AS, Gusarov I, Rafikov R, Lopez LE, Shat<strong>al</strong>in K,<br />
Kreneva RA, Perumov DA, Nudler E: Sensing sm<strong>al</strong>l molecules by<br />
nascent RNA: a mechanism to control transcription in<br />
bacteria. Cell 2002, 111:747-756.<br />
45. Wickiser JK, Cheah MT, Breaker RR, Crothers DM: The kin<strong>et</strong>ics<br />
of ligand binding by an adenine-sensing riboswitch.<br />
Biochemistry 2005, 44:13404-13414.<br />
46.<br />
<br />
Wickiser JK, Winkler WC, Breaker RR, Crothers DM: The speed of<br />
RNA transcription and m<strong>et</strong>abolite binding kin<strong>et</strong>ics operate an<br />
FMN riboswitch. Mol Cell 2005, 18:49-60.<br />
The rates of riboswitch transcription, the influence of transcription<strong>al</strong><br />
pause sites and the association rates of FMN are studied, showing that<br />
the FMN riboswitch is under kin<strong>et</strong>ic rather than thermodynamic control.<br />
47. Grundy FJ, Henkin TM: Regulation of gene expression by<br />
effectors that bind to RNA. <strong>Curr</strong> <strong>Opin</strong> Microbiol 2004,<br />
7:126-131.<br />
48. Cromie MJ, Shi Y, Latifi T, Groisman EA: An RNA sensor for<br />
intracellular Mg(2+). Cell 2006, 125:71-84.<br />
49. Chowdhury S, Maris C, Allain FH, Narberhaus F: Molecular<br />
basis for temperature sensing by an RNA thermom<strong>et</strong>er.<br />
EMBO J 2006, 25:2487-2497.<br />
50. Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-<br />
Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS <strong>et</strong> <strong>al</strong>.:<br />
Cryst<strong>al</strong>lography & NMR system: a new software suite for<br />
macromolecular structure d<strong>et</strong>ermination. Acta Cryst<strong>al</strong>logr D<br />
<strong>Biol</strong> Cryst<strong>al</strong>logr 1998, 54:905-921.<br />
51. DeLano WL: The PyMOL Molecular Graphics System. San Carlos,<br />
CA: DeLano Scientific; 2002.<br />
www.sciencedirect.com <strong>Curr</strong>ent <strong>Opin</strong>ion in <strong>Struct</strong>ur<strong>al</strong> <strong>Biol</strong>ogy <strong>2007</strong>, 17:273–279