Spin-density distribution of the p-O2 N·C6 F4 ·CNSSN free radical by ...
Spin-density distribution of the p-O2 N·C6 F4 ·CNSSN free radical by ...
Spin-density distribution of the p-O2 N·C6 F4 ·CNSSN free radical by ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
scientifichighlights modelling<br />
<strong>Spin</strong>-<strong>density</strong> <strong>distribution</strong><br />
<strong>of</strong> <strong>the</strong> p-O 2<br />
N·C 6<br />
F 4<strong>·CNSSN</strong><br />
<strong>free</strong> <strong>radical</strong> <strong>by</strong> experiment<br />
and ab initio calculations.<br />
The X·C 6<br />
F 4<br />
CNSSN• <strong>radical</strong>s, where X is an anion, are very promising as<br />
building blocks in <strong>the</strong> search for organic magnets <strong>free</strong> <strong>of</strong> metallic compounds,<br />
a field <strong>of</strong> increasing interest in <strong>the</strong> scientific community. Knowledge<br />
<strong>of</strong> <strong>the</strong> spin-<strong>density</strong> <strong>distribution</strong> in <strong>the</strong> CNSSN• dithiadiazolyl <strong>radical</strong><br />
ring (DTDA for short) constitutes a major step towards <strong>the</strong> understanding<br />
<strong>of</strong> <strong>the</strong> magnetic and electronic properties <strong>of</strong> <strong>the</strong> rich magnetism<br />
<strong>of</strong> DTDA derivatives. The O 2<br />
N·C 6<br />
F 4<br />
CNSSN• <strong>radical</strong> was chosen as <strong>the</strong><br />
most favorable DTDA derivative to study <strong>the</strong> spin <strong>distribution</strong> in this kind<br />
<strong>of</strong> <strong>free</strong> <strong>radical</strong> <strong>by</strong> polarized-neutron diffraction with support <strong>by</strong> ab initio<br />
calculations based on <strong>density</strong> functional <strong>the</strong>ory. Both <strong>the</strong> experiment<br />
and <strong>the</strong> ab initio calculations show that almost all <strong>the</strong> spin <strong>density</strong> is localized<br />
on <strong>the</strong> sulfur and nitrogen atoms <strong>of</strong> <strong>the</strong> DTDA ring with a small negative<br />
spin <strong>density</strong> on <strong>the</strong> carbon atom <strong>of</strong> <strong>the</strong> DTDA ring and negligible<br />
spin <strong>density</strong> over <strong>the</strong> rest <strong>of</strong> <strong>the</strong> <strong>radical</strong>.<br />
In recent years, <strong>the</strong>re has been<br />
considerable interest in <strong>the</strong> study <strong>of</strong> <strong>the</strong><br />
magnetic behaviour <strong>of</strong> <strong>free</strong> <strong>radical</strong>s [1].<br />
Part <strong>of</strong> this interest has focused on <strong>the</strong><br />
development and study <strong>of</strong> organic materials<br />
that exhibit spontaneous magnetization<br />
on decreasing temperature in<br />
order to develop organic magnets <strong>free</strong><br />
<strong>of</strong> metallic elements. Such materials can<br />
exhibit peculiar and probably unprecedented<br />
properties not shown <strong>by</strong> <strong>the</strong> traditional<br />
inorganic magnetic materials<br />
based on metallic or ionic lattices: low<br />
<strong>density</strong>, transparency, photo-responsiveness,<br />
electrical insulation, biocompatibility,<br />
low-temperature fabrication, easy<br />
processability etc. Fur<strong>the</strong>rmore, a most<br />
interesting characteristic <strong>of</strong> <strong>the</strong>se materials<br />
is <strong>the</strong> possibility <strong>of</strong> performing<br />
slight chemical modifications to change<br />
<strong>the</strong>se physical properties.<br />
The dithiadiazolyl <strong>radical</strong>s X·C 6<br />
F 4<br />
CNSSN•<br />
(X = Br, O 2<br />
N, CN,…) present a very interesting<br />
variety <strong>of</strong> magnetic behaviours.<br />
The p-NC-C 6<br />
F 4<br />
CNSSN• <strong>radical</strong> has been<br />
reported as a weak ferromagnet with<br />
an ordering temperature <strong>of</strong> 36 K [2], <strong>the</strong><br />
highest observed in a purely organic<br />
N1<br />
N1<br />
O1<br />
C1<br />
F1<br />
F1<br />
C2<br />
C2<br />
C3<br />
C3<br />
F2<br />
F2<br />
C4<br />
J. Luzon, G.J. McIntyre<br />
and M.R. Johnson (ILL)<br />
J. Campo and F. Palacio<br />
(ICMA, Zaragoza)<br />
C.M. Pask and J.M. Rawson<br />
(University <strong>of</strong> Cambridge, UK)<br />
magnet to date. By replacing <strong>the</strong> CN<br />
group <strong>by</strong> Br a paramagnetic system<br />
possessing very weak antiferromagnetic<br />
interactions is obtained. And,<br />
finally, if <strong>the</strong> CN group is substituted <strong>by</strong><br />
NO 2<br />
to give <strong>the</strong> p-O 2<br />
N·C 6<br />
F 4<br />
CNSSN• <strong>radical</strong>,<br />
ferromagnetic ordering is observed<br />
below T c<br />
= 1.3 K [3].<br />
These different magnetic behaviours<br />
are due to <strong>the</strong> change in <strong>the</strong> molecular<br />
packing and, consequently, a substantial<br />
modification <strong>of</strong> <strong>the</strong> magnetic<br />
interaction pathways. Knowledge <strong>of</strong><br />
<strong>the</strong> spin-<strong>density</strong> <strong>distribution</strong> in <strong>the</strong><br />
dithiadiazolyl <strong>radical</strong> ring allows us a<br />
better understanding <strong>of</strong> <strong>the</strong> rich magnetism<br />
<strong>of</strong> DTDA derivatives and hence<br />
helps in <strong>the</strong> design <strong>of</strong> new organic ferromagnets<br />
with higher T C<br />
based on <strong>the</strong><br />
DTDA ring.<br />
In <strong>the</strong> work reported here we have<br />
studied <strong>the</strong> spin-<strong>density</strong> <strong>distribution</strong><br />
in <strong>the</strong> p-O 2<br />
N·C 6<br />
F 4<br />
CNSSN• <strong>radical</strong> <strong>by</strong> <strong>the</strong><br />
complementary techniques <strong>of</strong> polarized-neutron<br />
diffraction and ab initio<br />
calculations.<br />
-1.20<br />
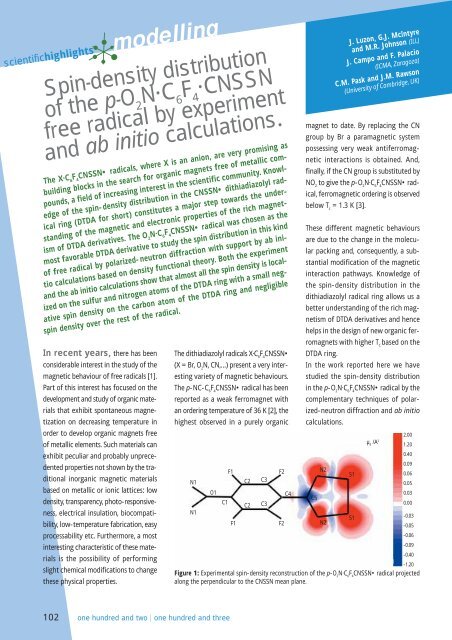
Figure 1: Experimental spin-<strong>density</strong> reconstruction <strong>of</strong> <strong>the</strong> p-O 2<br />
N·C 6<br />
F 4<br />
CNSSN• <strong>radical</strong> projected<br />
along <strong>the</strong> perpendicular to <strong>the</strong> CNSSN mean plane.<br />
C5<br />
N2<br />
N2<br />
S1<br />
S1<br />
µ B<br />
/A 2<br />
2.00<br />
1.20<br />
0.40<br />
0.09<br />
0.06<br />
0.05<br />
0.03<br />
0.00<br />
-0.03<br />
-0.05<br />
-0.06<br />
-0.09<br />
-0.40<br />
102 one hundred and two | one hundred and three
µB N2 S1 C5 C1 C2 C3 C4 F2<br />
BLYP-molecule 0.226 0.308 0.065 -0.003 0.001 -0.004 0.005 0.000<br />
PW91-molecule 0.220 0.312 -0.061 -0.003 0.001 -0.004 0.002 0.000<br />
VWM-molecule 0.225 0.310 -0.062 -0.003 0.001 -0.004 0.002 0.000<br />
BLYP-crystal 0.214 0.317 -0.061 -0.003 0.002 -0.004 0.004 0.001<br />
This experiment 0.247 0.281 -0.055<br />
Table 1: Ab initio spin-<strong>density</strong> populations in <strong>the</strong> p-O 2<br />
N·C 6<br />
F 4<br />
CNSSN• <strong>radical</strong>. See figure 1 for <strong>the</strong><br />
atomic labelling.<br />
The polarized-neutron diffraction<br />
experiment was performed on <strong>the</strong> D3<br />
lifting-counter diffractometer at <strong>the</strong><br />
ILL at 1.5 K in an applied field <strong>of</strong> 9 T.<br />
A multipolar model <strong>of</strong> <strong>the</strong> spin <strong>density</strong><br />
(figure 1) was fitted to <strong>the</strong> measured<br />
flipping ratios. Almost all <strong>the</strong> spin <strong>density</strong><br />
is localized in <strong>the</strong> nitrogen and sulfur<br />
p orbitals perpendicular to <strong>the</strong> DTDA<br />
ring (0.281 µ B<br />
and 0.247 µ B<br />
, respectively).<br />
These orbitals generate <strong>the</strong> Single<br />
Occupied Molecular Orbital (SOMO).<br />
Some negative <strong>density</strong> is observed<br />
between <strong>the</strong> sulfur and nitrogen atoms<br />
that can be due to covalence and polarization<br />
effects. In addition, a small negative<br />
<strong>density</strong> is observed (-0.055 µ B<br />
) on<br />
<strong>the</strong> carbon site <strong>of</strong> <strong>the</strong> DTDA ring due to<br />
polarization effects. The spin <strong>density</strong><br />
over <strong>the</strong> rest <strong>of</strong> <strong>the</strong> <strong>radical</strong> is below<br />
<strong>the</strong> limits <strong>of</strong> <strong>the</strong> experimental accuracy.<br />
To support <strong>the</strong> experimental modelling,<br />
ab initio calculations were performed<br />
at <strong>the</strong> generalized gradient<br />
approximation level, using several<br />
exchange-correlation functionals<br />
(PW91, BLYP, VWM) with a double<br />
numerical basis set including functions<br />
and numerical integrations, as implemented<br />
in <strong>the</strong> Dmol3 s<strong>of</strong>tware based on<br />
<strong>density</strong> functional <strong>the</strong>ory. Both periodic<br />
(crystal) and molecular calculations<br />
were made.<br />
The ab initio calculations (figure 2 and<br />
table I) confirm <strong>the</strong> experimental results<br />
and, in particular, <strong>the</strong>y show that <strong>the</strong><br />
negative spin <strong>density</strong> in <strong>the</strong> plane <strong>of</strong><br />
<strong>the</strong> DTDA ring is not a spurious effect <strong>of</strong><br />
<strong>the</strong> multipolar model used. This negative<br />
spin <strong>density</strong> could play a very important<br />
role in <strong>the</strong> ferromagnetic behavior <strong>of</strong> <strong>the</strong><br />
O 2<br />
N·C 6<br />
F 4<br />
CNSSN• <strong>radical</strong>. The spin populations<br />
in <strong>the</strong> rest <strong>of</strong> <strong>the</strong> molecule could<br />
also be obtained: in <strong>the</strong> carbon atoms<br />
<strong>the</strong>re are alternate negative and positive<br />
low spin <strong>density</strong> populations due to<br />
polarization effects. Only a suggestion<br />
<strong>of</strong> <strong>the</strong> latter was observed experimentally,<br />
which emphasizes <strong>the</strong> importance<br />
<strong>of</strong> combining experiment and ab initio<br />
calculations in such studies.<br />
Figure 2: <strong>Spin</strong>-<strong>density</strong> <strong>distribution</strong> <strong>of</strong> <strong>the</strong> p-O 2<br />
N·C 6<br />
F 4<br />
CNSSN• <strong>radical</strong> calculated with <strong>the</strong> Dmol3<br />
package (BLYP functional, molecular calculation). The red surface is <strong>the</strong> contour <strong>of</strong> 0.0002 µ B<br />
/Å 3<br />
and <strong>the</strong> blue one is <strong>the</strong> contour <strong>of</strong> –0.0002 µ B<br />
/Å 3 .<br />
References: [1] "Magnetic Properties <strong>of</strong> Organic Materials" Ed. By P. Lahti, Marcel Dekker Inc New York (1999)<br />
[2] F. Palacio, G. Antorrena, M. Castro, R. Burriel, J.M. Rawson, J.N.B. Smith, N. Bricklebank, J. Novoa and C. Ritter, Phys. Rev.<br />
Lett. 79 (1997) 2336-9<br />
[3] J. M. Rawson, C.M. Pask, F. Palacio, P. Oliete, C. Paulsen, A. Yamaguchi and R.D. Farley (To be published)<br />
103