Pharmacokinetics of Piperacillin-Tazobactam in Anuric Intensive ...
Pharmacokinetics of Piperacillin-Tazobactam in Anuric Intensive ...
Pharmacokinetics of Piperacillin-Tazobactam in Anuric Intensive ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1558 NOTES ANTIMICROB. AGENTS CHEMOTHER.<br />
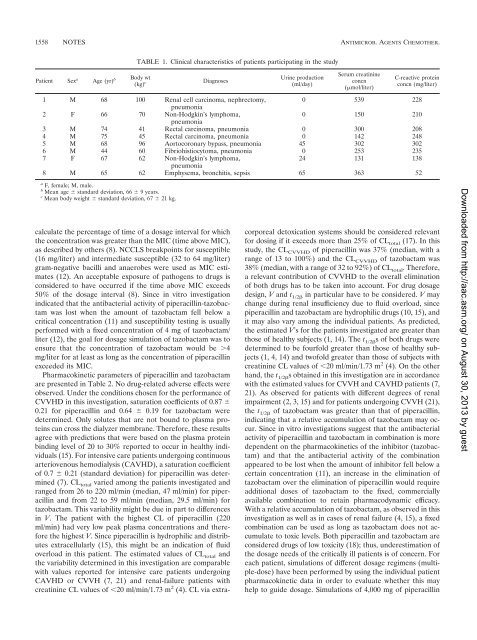
Patient Sex a Age (yr) b Body wt<br />
(kg) c<br />
TABLE 1. Cl<strong>in</strong>ical characteristics <strong>of</strong> patients participat<strong>in</strong>g <strong>in</strong> the study<br />
Diagnoses<br />
Ur<strong>in</strong>e production<br />
(ml/day)<br />
Serum creat<strong>in</strong><strong>in</strong>e<br />
concn<br />
(mol/liter)<br />
C-reactive prote<strong>in</strong><br />
concn (mg/liter)<br />
1 M 68 100 Renal cell carc<strong>in</strong>oma, nephrectomy,<br />
0 539 228<br />
pneumonia<br />
2 F 66 70 Non-Hodgk<strong>in</strong>’s lymphoma,<br />
0 150 210<br />
pneumonia<br />
3 M 74 41 Rectal carc<strong>in</strong>oma, pneumonia 0 300 208<br />
4 M 75 45 Rectal carc<strong>in</strong>oma, pneumonia 0 142 248<br />
5 M 68 96 Aortocoronary bypass, pneumonia 45 302 302<br />
6 M 44 60 Fibriohistiocytoma, pneumonia 0 253 235<br />
7 F 67 62 Non-Hodgk<strong>in</strong>’s lymphoma,<br />
24 131 138<br />
pneumonia<br />
8 M 65 62 Emphysema, bronchitis, sepsis 65 363 52<br />
a F, female; M, male.<br />
b Mean age standard deviation, 66 9 years.<br />
c Mean body weight standard deviation, 67 21 kg.<br />
calculate the percentage <strong>of</strong> time <strong>of</strong> a dosage <strong>in</strong>terval for which<br />
the concentration was greater than the MIC (time above MIC),<br />
as described by others (8). NCCLS breakpo<strong>in</strong>ts for susceptible<br />
(16 mg/liter) and <strong>in</strong>termediate susceptible (32 to 64 mg/liter)<br />
gram-negative bacilli and anaerobes were used as MIC estimates<br />
(12). An acceptable exposure <strong>of</strong> pathogens to drugs is<br />
considered to have occurred if the time above MIC exceeds<br />
50% <strong>of</strong> the dosage <strong>in</strong>terval (8). S<strong>in</strong>ce <strong>in</strong> vitro <strong>in</strong>vestigation<br />
<strong>in</strong>dicated that the antibacterial activity <strong>of</strong> piperacill<strong>in</strong>-tazobactam<br />
was lost when the amount <strong>of</strong> tazobactam fell below a<br />
critical concentration (11) and susceptibility test<strong>in</strong>g is usually<br />
performed with a fixed concentration <strong>of</strong> 4 mg <strong>of</strong> tazobactam/<br />
liter (12), the goal for dosage simulation <strong>of</strong> tazobactam was to<br />
ensure that the concentration <strong>of</strong> tazobactam would be 4<br />
mg/liter for at least as long as the concentration <strong>of</strong> piperacill<strong>in</strong><br />
exceeded its MIC.<br />
Pharmacok<strong>in</strong>etic parameters <strong>of</strong> piperacill<strong>in</strong> and tazobactam<br />
are presented <strong>in</strong> Table 2. No drug-related adverse effects were<br />
observed. Under the conditions chosen for the performance <strong>of</strong><br />
CVVHD <strong>in</strong> this <strong>in</strong>vestigation, saturation coefficients <strong>of</strong> 0.87 <br />
0.21 for piperacill<strong>in</strong> and 0.64 0.19 for tazobactam were<br />
determ<strong>in</strong>ed. Only solutes that are not bound to plasma prote<strong>in</strong>s<br />
can cross the dialyzer membrane. Therefore, these results<br />
agree with predictions that were based on the plasma prote<strong>in</strong><br />
b<strong>in</strong>d<strong>in</strong>g level <strong>of</strong> 20 to 30% reported to occur <strong>in</strong> healthy <strong>in</strong>dividuals<br />
(15). For <strong>in</strong>tensive care patients undergo<strong>in</strong>g cont<strong>in</strong>uous<br />
arteriovenous hemodialysis (CAVHD), a saturation coefficient<br />
<strong>of</strong> 0.7 0.21 (standard deviation) for piperacill<strong>in</strong> was determ<strong>in</strong>ed<br />
(7). CL total varied among the patients <strong>in</strong>vestigated and<br />
ranged from 26 to 220 ml/m<strong>in</strong> (median, 47 ml/m<strong>in</strong>) for piperacill<strong>in</strong><br />
and from 22 to 59 ml/m<strong>in</strong> (median, 29.5 ml/m<strong>in</strong>) for<br />
tazobactam. This variability might be due <strong>in</strong> part to differences<br />
<strong>in</strong> V. The patient with the highest CL <strong>of</strong> piperacill<strong>in</strong> (220<br />
ml/m<strong>in</strong>) had very low peak plasma concentrations and therefore<br />
the highest V. S<strong>in</strong>ce piperacill<strong>in</strong> is hydrophilic and distributes<br />
extracellularly (15), this might be an <strong>in</strong>dication <strong>of</strong> fluid<br />
overload <strong>in</strong> this patient. The estimated values <strong>of</strong> CL total and<br />
the variability determ<strong>in</strong>ed <strong>in</strong> this <strong>in</strong>vestigation are comparable<br />
with values reported for <strong>in</strong>tensive care patients undergo<strong>in</strong>g<br />
CAVHD or CVVH (7, 21) and renal-failure patients with<br />
creat<strong>in</strong><strong>in</strong>e CL values <strong>of</strong> 20 ml/m<strong>in</strong>/1.73 m 2 (4). CL via extracorporeal<br />
detoxication systems should be considered relevant<br />
for dos<strong>in</strong>g if it exceeds more than 25% <strong>of</strong> CL total (17). In this<br />
study, the CL CVVHD <strong>of</strong> piperacill<strong>in</strong> was 37% (median, with a<br />
range <strong>of</strong> 13 to 100%) and the CL CVVHD <strong>of</strong> tazobactam was<br />
38% (median, with a range <strong>of</strong> 32 to 92%) <strong>of</strong> CL total . Therefore,<br />
a relevant contribution <strong>of</strong> CVVHD to the overall elim<strong>in</strong>ation<br />
<strong>of</strong> both drugs has to be taken <strong>in</strong>to account. For drug dosage<br />
design, V and t 1/2 <strong>in</strong> particular have to be considered. V may<br />
change dur<strong>in</strong>g renal <strong>in</strong>sufficiency due to fluid overload, s<strong>in</strong>ce<br />
piperacill<strong>in</strong> and tazobactam are hydrophilic drugs (10, 15), and<br />
it may also vary among the <strong>in</strong>dividual patients. As predicted,<br />
the estimated V’s for the patients <strong>in</strong>vestigated are greater than<br />
those <strong>of</strong> healthy subjects (1, 14). The t 1/2 s <strong>of</strong> both drugs were<br />
determ<strong>in</strong>ed to be fourfold greater than those <strong>of</strong> healthy subjects<br />
(1, 4, 14) and tw<strong>of</strong>old greater than those <strong>of</strong> subjects with<br />
creat<strong>in</strong><strong>in</strong>e CL values <strong>of</strong> 20 ml/m<strong>in</strong>/1.73 m 2 (4). On the other<br />
hand, the t 1/2 s obta<strong>in</strong>ed <strong>in</strong> this <strong>in</strong>vestigation are <strong>in</strong> accordance<br />
with the estimated values for CVVH and CAVHD patients (7,<br />
21). As observed for patients with different degrees <strong>of</strong> renal<br />
impairment (2, 3, 15) and for patients undergo<strong>in</strong>g CVVH (21),<br />
the t 1/2 <strong>of</strong> tazobactam was greater than that <strong>of</strong> piperacill<strong>in</strong>,<br />
<strong>in</strong>dicat<strong>in</strong>g that a relative accumulation <strong>of</strong> tazobactam may occur.<br />
S<strong>in</strong>ce <strong>in</strong> vitro <strong>in</strong>vestigations suggest that the antibacterial<br />
activity <strong>of</strong> piperacill<strong>in</strong> and tazobactam <strong>in</strong> comb<strong>in</strong>ation is more<br />
dependent on the pharmacok<strong>in</strong>etics <strong>of</strong> the <strong>in</strong>hibitor (tazobactam)<br />
and that the antibacterial activity <strong>of</strong> the comb<strong>in</strong>ation<br />
appeared to be lost when the amount <strong>of</strong> <strong>in</strong>hibitor fell below a<br />
certa<strong>in</strong> concentration (11), an <strong>in</strong>crease <strong>in</strong> the elim<strong>in</strong>ation <strong>of</strong><br />
tazobactam over the elim<strong>in</strong>ation <strong>of</strong> piperacill<strong>in</strong> would require<br />
additional doses <strong>of</strong> tazobactam to the fixed, commercially<br />
available comb<strong>in</strong>ation to reta<strong>in</strong> pharmacodynamic efficacy.<br />
With a relative accumulation <strong>of</strong> tazobactam, as observed <strong>in</strong> this<br />
<strong>in</strong>vestigation as well as <strong>in</strong> cases <strong>of</strong> renal failure (4, 15), a fixed<br />
comb<strong>in</strong>ation can be used as long as tazobactam does not accumulate<br />
to toxic levels. Both piperacill<strong>in</strong> and tazobactam are<br />
considered drugs <strong>of</strong> low toxicity (18); thus, underestimation <strong>of</strong><br />
the dosage needs <strong>of</strong> the critically ill patients is <strong>of</strong> concern. For<br />
each patient, simulations <strong>of</strong> different dosage regimens (multiple-dose)<br />
have been performed by us<strong>in</strong>g the <strong>in</strong>dividual patient<br />
pharmacok<strong>in</strong>etic data <strong>in</strong> order to evaluate whether this may<br />
help to guide dosage. Simulations <strong>of</strong> 4,000 mg <strong>of</strong> piperacill<strong>in</strong><br />
Downloaded from http://aac.asm.org/ on August 30, 2013 by guest