Planetary Geochemistry: Solar System Formation
Planetary Geochemistry: Solar System Formation
Planetary Geochemistry: Solar System Formation
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Planetary</strong> <strong>Geochemistry</strong>:<br />
<strong>Solar</strong> <strong>System</strong> <strong>Formation</strong>
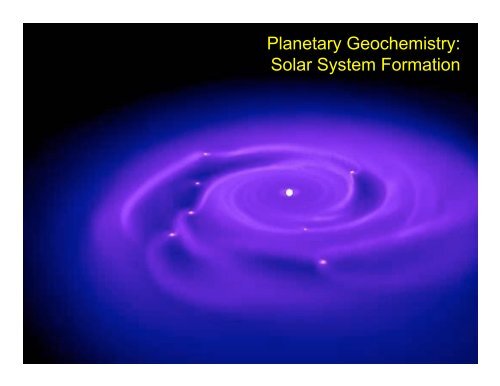
Origin of the <strong>Solar</strong> <strong>System</strong><br />
! Initial Condition: <strong>Solar</strong> Nebula<br />
• Diffuse mass of interstellar gas/dust<br />
• Began contraction ~6 billion years ago<br />
• Seeded with heavy elements via ancestral supernovae events<br />
! Triggering Event?<br />
• A passing star?<br />
• Thought to be common initiator of solar system formation<br />
• A nearby supernova?<br />
• Meteorites contain more 129 Xe and 26 Mg than they should<br />
• These isotopes can only form in large amounts through radiogenic processes<br />
129 I => 129 Xe + !- T 1/2 = 17 My<br />
26 Al => 26 Mg + !- T 1/2 = 0.72 My<br />
• For daughters to be in modern meteorites, parent isotopes had to form just<br />
before solar system<br />
• Only likely source is a nearby supernova just before system condensation
<strong>Solar</strong> Nebula <strong>Formation</strong><br />
! Contraction<br />
• Conservation of angular momentum dictates contracting cloud begins to rotate<br />
• Rotation leads to formation of central mass, surrounded by disk<br />
! Central Disk<br />
• Temperature/Pressure gradients ensue, resulting in chemical differentiation<br />
• 1000 - 2000 K, 0.01 - 0.1 atm at center<br />
• 40 K, 10 -7 atm near edge<br />
• Condensation - volatilization followed by condensation, OR<br />
• Evaporation - partial melting, evaporation, refractory particles remain solid<br />
• Likely a combination of both…
<strong>Solar</strong> Nebula<br />
! Protosun<br />
• Begins H fusion
<strong>Solar</strong> Nebula
Outer <strong>System</strong><br />
Inner <strong>System</strong>
Planet <strong>Formation</strong><br />
! Planets - formed from accreting planetesimals<br />
• Terrestrial planets<br />
• Mercury, Venus, Earth, Mars<br />
• Comprised chiefly of elements with low vapor pressures<br />
• Solid, rocky objects, relatively low volatile contents<br />
• Gas Giants (Jovian(<br />
planets)<br />
• Jupiter, Saturn, Uranus, Neptune<br />
• Essentially solar in composition (H, He)<br />
• May have condensed liquid or solid cores (?)<br />
• Liquid metallic H? Diamond?<br />
• Bode’s Law<br />
• Distances of the planets from the Sun obey a simple arithmetic relation<br />
a = 0.4 + 0.3(2 n-2 ) where n = 2, 3, 4…<br />
• Where a = distance in Astronomical Units (AU) - mean distance from Sun to<br />
Earth (93(<br />
million miles, 1.5 x 10 8 km)<br />
• Likely a result of orbital resonance effects
Planet <strong>Formation</strong> (cont’d)<br />
! Mass Distribution<br />
• 99.87% of solar system mass is in Sun<br />
• 0.13% is all the rest of the solar system combined, most of which is Jupiter<br />
• 71% of planetary mass is in Jupiter<br />
• Most of the remaining 29% is in Saturn, Uranus, Neptune<br />
• 0.44% of planetary mass is in the terrestrial planets<br />
• 50.3% in Earth<br />
• 40.9% in Venus<br />
• 5.4% in Mars<br />
• 2.8% in Mercury
<strong>Formation</strong> of <strong>Planetary</strong> Materials<br />
! Required first the condensation of rocky material in the solar nebula<br />
• Nickel-Iron<br />
• Likely the first to condense in nebula<br />
• Minerals kamacite and taenite<br />
• Highest temperature of condensation (~1400 K)<br />
• Silicates, Other Minerals<br />
• Early condensation of metallic silicon (Si)<br />
• Condensed in nebula at 1300 - 1000 K<br />
• Higest temperature condensates included perovskite (CaTiO 3 )<br />
• Common mineral condensates were enstatite (MgSiO 3 ), feldspars<br />
• Solid-solid reactions<br />
• At slightly lower temperatures (700 - 500 K), solids reacted to form alteration<br />
products<br />
• Troilite (FeS) from reaction of sulfur with kamacite/taenite<br />
taenite<br />
• Olivine from reaction of enstatite with kamacite/taenite<br />
taenite<br />
• FeO (wustite) from reaction of oxygen with kamacite/taenite<br />
taenite