GaBi Paper Clip Tutorial - GaBi Software
GaBi Paper Clip Tutorial - GaBi Software GaBi Paper Clip Tutorial - GaBi Software
Appendix A Appendix A 6 Photochemical Ozone Creation Potential (POCP) Despite playing a protective role in the stratosphere, at ground-level ozone is classified as a damaging trace gas. Photochemical ozone production in the troposphere, also known as summer smog, is suspected to damage vegetation and material. High concentrations of ozone are toxic to humans. Radiation from the sun and the presence of nitrogen oxides and hydrocarbons incur complex chemical reactions, producing aggressive reaction products, one of which is ozone. Nitrogen oxides alone do not cause high ozone concentration levels. Hydrocarbon emissions occur from incomplete combustion, in conjunction with petrol (storage, turnover, refuelling etc.) or from solvents. High concentrations of ozone arise when the temperature is high, humidity is low, when air is relatively static and when there are high concentrations of hydrocarbons. Today it is assumed that the existence of NO and CO reduces the accumulated ozone to NO2, CO2 and O2. This means, that high concentrations of ozone do not often occur near hydrocarbon emission sources. Higher ozone concentrations more commonly arise in areas of clean air, such as forests, where there is less NO and CO (Figure A 4). In Life Cycle Assessments, photochemical ozone creation potential (POCP) is referred to in ethyleneequivalents (C2H4-Äq.). When analyzing, it’s important to remember that the actual ozone concentration is strongly influenced by the weather and by the characteristics of the local conditions. Hydrocarbons Nitrogen oxides Appendix A 7 Ozone Depletion Potential (ODP) Figure A 4: Photochemical Ozone Creation Potential (ISO 14044:2006) Ozone is created in the stratosphere by the disassociation of oxygen atoms that are exposed to short-wave UV-light. This leads to the formation of the so-called ozone layer in the stratosphere (15 - 50 km high). About 10 % of this ozone reaches the troposphere through mixing processes. In spite of its minimal concentration, the ozone layer is essential for life on earth. Ozone absorbs the short-wave UV-radiation and releases it in longer wavelengths. As a result, only a small part of the UV-radiation reaches the earth. Anthropogenic emissions deplete ozone. This is well-known from reports on the hole in the ozone layer. The hole is currently confined to the region above Antarctica, however another ozone depletion can be identified, albeit not to the same extent, over the midlatitudes (e.g. Europe). The substances which have a depleting effect on the ozone can essentially be divided into two groups; the fluorine-chlorine-hydrocarbons (CFCs) and the nitrogen oxides (NOX). Figure A 5 depicts the procedure of ozone depletion. One effect of ozone depletion is the warming of the earth's surface. The sensitivity of humans, animals and plants to UV-B and UV-A radiation is of particular importance. Possible 72 Ozone Dry and warm climate Hydrocarbons Nitrogen oxides
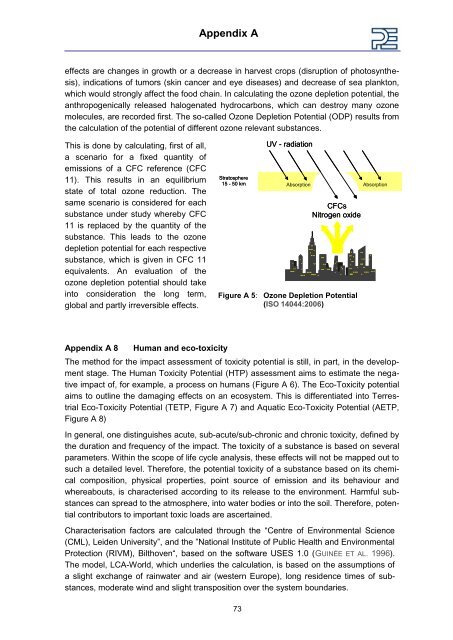
Appendix A effects are changes in growth or a decrease in harvest crops (disruption of photosynthesis), indications of tumors (skin cancer and eye diseases) and decrease of sea plankton, which would strongly affect the food chain. In calculating the ozone depletion potential, the anthropogenically released halogenated hydrocarbons, which can destroy many ozone molecules, are recorded first. The so-called Ozone Depletion Potential (ODP) results from the calculation of the potential of different ozone relevant substances. This is done by calculating, first of all, a scenario for a fixed quantity of emissions of a CFC reference (CFC 11). This results in an equilibrium state of total ozone reduction. The same scenario is considered for each substance under study whereby CFC 11 is replaced by the quantity of the substance. This leads to the ozone depletion potential for each respective substance, which is given in CFC 11 equivalents. An evaluation of the ozone depletion potential should take into consideration the long term, global and partly irreversible effects. Appendix A 8 Human and eco-toxicity Stratosphere 15 - 50 km Absorption Absorption Figure A 5: Ozone Depletion Potential (ISO 14044:2006) The method for the impact assessment of toxicity potential is still, in part, in the development stage. The Human Toxicity Potential (HTP) assessment aims to estimate the negative impact of, for example, a process on humans (Figure A 6). The Eco-Toxicity potential aims to outline the damaging effects on an ecosystem. This is differentiated into Terrestrial Eco-Toxicity Potential (TETP, Figure A 7) and Aquatic Eco-Toxicity Potential (AETP, Figure A 8) In general, one distinguishes acute, sub-acute/sub-chronic and chronic toxicity, defined by the duration and frequency of the impact. The toxicity of a substance is based on several parameters. Within the scope of life cycle analysis, these effects will not be mapped out to such a detailed level. Therefore, the potential toxicity of a substance based on its chemical composition, physical properties, point source of emission and its behaviour and whereabouts, is characterised according to its release to the environment. Harmful substances can spread to the atmosphere, into water bodies or into the soil. Therefore, potential contributors to important toxic loads are ascertained. Characterisation factors are calculated through the “Centre of Environmental Science (CML), Leiden University”, and the ”National Institute of Public Health and Environmental Protection (RIVM), Bilthoven“, based on the software USES 1.0 (GUINÉE ET AL. 1996). The model, LCA-World, which underlies the calculation, is based on the assumptions of a slight exchange of rainwater and air (western Europe), long residence times of substances, moderate wind and slight transposition over the system boundaries. 73 UV - radiation CFCs Nitrogen oxide
- Page 21 and 22: Conducting Life Cycle Assessments I
- Page 23 and 24: Conducting Life Cycle Assessments F
- Page 25 and 26: 2.4 Interpretation Conducting Life
- Page 27 and 28: Conducting Life Cycle Assessments 3
- Page 29 and 30: If so, you can skip this step. Proc
- Page 31 and 32: 3.5 Flows Procedure Perhaps the mos
- Page 33 and 34: 3.6 Starting a project Procedure Yo
- Page 35 and 36: There are 2 ways to do this in GaBi
- Page 37 and 38: Procedure A window opens where you
- Page 39 and 40: 3.13 Parameters Procedure Here you
- Page 41 and 42: Procedure When you select a flow fr
- Page 43 and 44: Procedure 38. Type in the column
- Page 45 and 46: Procedure We do this to specify the
- Page 47 and 48: Procedure 49. Double click on the
- Page 49 and 50: Procedure You can also use the auto
- Page 51 and 52: 3.25 Resizing process boxes Procedu
- Page 53 and 54: Procedure 62. Connect the steel wir
- Page 55 and 56: 3.27 Adding a plan Procedure We wil
- Page 57 and 58: 3.29 Adding comments Procedure You
- Page 59 and 60: Procedure You can drill down into t
- Page 61 and 62: Procedure 97. Double click on the
- Page 63 and 64: Procedure As you see, only the flow
- Page 65 and 66: Procedure By right clicking on a co
- Page 67 and 68: 3.39 Exporting results Procedure If
- Page 69 and 70: Appendix A Appendix A Description o
- Page 71: The acidification potential is give
- Page 75: Appendix A Appendix A 9 Abiotic Dep
Appendix A<br />
effects are changes in growth or a decrease in harvest crops (disruption of photosynthesis),<br />
indications of tumors (skin cancer and eye diseases) and decrease of sea plankton,<br />
which would strongly affect the food chain. In calculating the ozone depletion potential, the<br />
anthropogenically released halogenated hydrocarbons, which can destroy many ozone<br />
molecules, are recorded first. The so-called Ozone Depletion Potential (ODP) results from<br />
the calculation of the potential of different ozone relevant substances.<br />
This is done by calculating, first of all,<br />
a scenario for a fixed quantity of<br />
emissions of a CFC reference (CFC<br />
11). This results in an equilibrium<br />
state of total ozone reduction. The<br />
same scenario is considered for each<br />
substance under study whereby CFC<br />
11 is replaced by the quantity of the<br />
substance. This leads to the ozone<br />
depletion potential for each respective<br />
substance, which is given in CFC 11<br />
equivalents. An evaluation of the<br />
ozone depletion potential should take<br />
into consideration the long term,<br />
global and partly irreversible effects.<br />
Appendix A 8 Human and eco-toxicity<br />
Stratosphere<br />
15 - 50 km Absorption Absorption<br />
Figure A 5: Ozone Depletion Potential<br />
(ISO 14044:2006)<br />
The method for the impact assessment of toxicity potential is still, in part, in the development<br />
stage. The Human Toxicity Potential (HTP) assessment aims to estimate the negative<br />
impact of, for example, a process on humans (Figure A 6). The Eco-Toxicity potential<br />
aims to outline the damaging effects on an ecosystem. This is differentiated into Terrestrial<br />
Eco-Toxicity Potential (TETP, Figure A 7) and Aquatic Eco-Toxicity Potential (AETP,<br />
Figure A 8)<br />
In general, one distinguishes acute, sub-acute/sub-chronic and chronic toxicity, defined by<br />
the duration and frequency of the impact. The toxicity of a substance is based on several<br />
parameters. Within the scope of life cycle analysis, these effects will not be mapped out to<br />
such a detailed level. Therefore, the potential toxicity of a substance based on its chemical<br />
composition, physical properties, point source of emission and its behaviour and<br />
whereabouts, is characterised according to its release to the environment. Harmful substances<br />
can spread to the atmosphere, into water bodies or into the soil. Therefore, potential<br />
contributors to important toxic loads are ascertained.<br />
Characterisation factors are calculated through the “Centre of Environmental Science<br />
(CML), Leiden University”, and the ”National Institute of Public Health and Environmental<br />
Protection (RIVM), Bilthoven“, based on the software USES 1.0 (GUINÉE ET AL. 1996).<br />
The model, LCA-World, which underlies the calculation, is based on the assumptions of<br />
a slight exchange of rainwater and air (western Europe), long residence times of substances,<br />
moderate wind and slight transposition over the system boundaries.<br />
73<br />
UV - radiation<br />
CFCs<br />
Nitrogen oxide