2. ENVIRONMENTAL ChEMISTRy & TEChNOLOGy 2.1. Lectures
2. ENVIRONMENTAL ChEMISTRy & TEChNOLOGy 2.1. Lectures
2. ENVIRONMENTAL ChEMISTRy & TEChNOLOGy 2.1. Lectures
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chem. Listy, 102, s265–s1311 (2008) Environmental Chemistry & Technology<br />
tion of zeolite was quartz 85 %, kaolinite 8 %, mica 6 % and<br />
iron minerals 3–5 %.<br />
Table III<br />
Chemical composition of quartz sands<br />
Components SiO 2 Al 2 O 3 Fe 2 O 3 MgO na 2 O<br />
% wt. 96.1 1.7 0.4 0.05 0.03<br />
C a l c i t e<br />
The natural materials, calcite was obtained from Horné<br />
Srnie location in Slovakia. The mineralogical composition of<br />
rock was calcite 80 %, quartz 5 %, kaolinite 2 %, plagioclase<br />
0.5 %, and iron minerals 0.7 %.<br />
Table IV<br />
Chemical composition of calcite<br />
Components SiO 2 Al 2 O 3 Fe 2 O 3 MgO na 2 O<br />
% wt. 11.8 3.9 1.2 0.8 0.05<br />
M a g n e z i t e<br />
The fired material of magnezite was obtained from Jelšava<br />
location in Slovakia. The mineralogical composition of<br />
sample was periclase 98 %.<br />
Table V<br />
Chemical composition of fired magnezite<br />
Components SiO 2 CaO Fe 2 O 3 MgO K 2 O<br />
% wt. 0.02 0.11 0.01 97.3 0.06<br />
Results<br />
The individual mineral phases of silicates (zeolite, bentonite,<br />
quartz sands) and carbonates (magnezite, calcite) can<br />
control the efficiency of biofiltration when sorption and precipitation<br />
of metals occurs. Moreover, in both cases, Zn, Cu,<br />
Pb were accumulated at the mineral and cell surface as precipitates.<br />
An important parameter for packing material is the<br />
removal capacity of the minerals.<br />
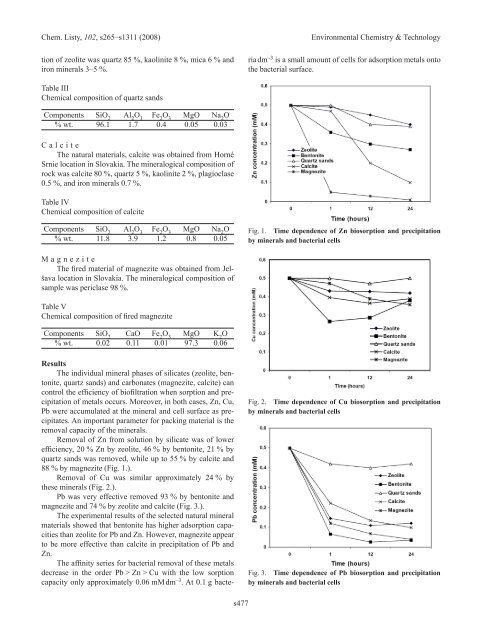
Removal of Zn from solution by silicate was of lower<br />
efficiency, 20 % Zn by zeolite, 46 % by bentonite, 21 % by<br />
quartz sands was removed, while up to 55 % by calcite and<br />
88 % by magnezite (Fig. 1.).<br />
Removal of Cu was similar approximately 24 % by<br />
these minerals (Fig. <strong>2.</strong>).<br />
Pb was very effective removed 93 % by bentonite and<br />
magnezite and 74 % by zeolite and calcite (Fig. 3.).<br />
The experimental results of the selected natural mineral<br />
materials showed that bentonite has higher adsorption capacities<br />
than zeolite for Pb and Zn. However, magnezite appear<br />
to be more effective than calcite in precipitation of Pb and<br />
Zn.<br />
The affinity series for bacterial removal of these metals<br />
decrease in the order Pb > Zn > Cu with the low sorption<br />
capacity only approximately 0.06 mM dm –3 . At 0.1 g bacte-<br />
s477<br />
ria dm –3 is a small amount of cells for adsorption metals onto<br />
the bacterial surface.<br />
Fig. 1. Time dependence of Zn biosorption and precipitation<br />
by minerals and bacterial cells<br />
Fig. <strong>2.</strong> Time dependence of Cu biosorption and precipitation<br />
by minerals and bacterial cells<br />
Fig. 3. Time dependence of Pb biosorption and precipitation<br />
by minerals and bacterial cells