2. ENVIRONMENTAL ChEMISTRy & TEChNOLOGy 2.1. Lectures
2. ENVIRONMENTAL ChEMISTRy & TEChNOLOGy 2.1. Lectures
2. ENVIRONMENTAL ChEMISTRy & TEChNOLOGy 2.1. Lectures
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chem. Listy, 102, s265–s1311 (2008) Environmental Chemistry & Technology<br />
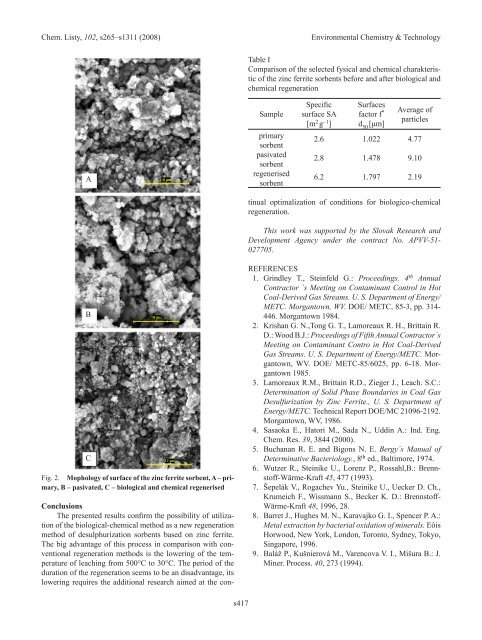
A<br />
B<br />
C<br />
Fig. <strong>2.</strong> Mophology of surface of the zinc ferrite sorbent, A – primary,<br />
b – pasivated, C – biological and chemical regenerised<br />
Conclusions<br />
The presented results confirm the possibility of utilization<br />
of the biological-chemical method as a new regeneration<br />
method of desulphurization sorbents based on zinc ferrite.<br />
The big advantage of this process in comparison with conventional<br />
regeneration methods is the lowering of the temperature<br />
of leaching from 500°C to 30°C. The period of the<br />
duration of the regeneration seems to be an disadvantage, its<br />
lowering requires the additional research aimed at the con-<br />
s417<br />
Table I<br />
Comparison of the selected fysical and chemical charakteristic<br />
of the zinc ferrite sorbents before and after biological and<br />
chemical regeneration<br />
Specific Surfaces<br />
Average of<br />
Sample surface SA factor f *<br />
particles<br />
[m2 g –1 ] d50 [μm]<br />
primary<br />
sorbent<br />
pasivated<br />
sorbent<br />
regenerised<br />
sorbent<br />
<strong>2.</strong>6 1.022 4.77<br />
<strong>2.</strong>8 1.478 9.10<br />
6.2 1.797 <strong>2.</strong>19<br />
tinual optimalization of conditions for biologico-chemical<br />
regeneration.<br />
This work was supported by the Slovak Research and<br />
Development Agency under the contract No. APVV-51-<br />
027705.<br />
REFEREnCES<br />
1. Grindley T., Steinfeld G.: Proceedings. 4 th Annual<br />
Contractor ´s Meeting on Contaminant Control in Hot<br />
Coal-Derived Gas Streams. U. S. Department of Energy/<br />
METC. Morgantown, WV. DOE/ METC, 85-3, pp. 314-<br />
446. Morgantown 1984.<br />
<strong>2.</strong> Krishan G. n.,Tong G. T., Lamoreaux R. H., Brittain R.<br />
D.: Wood B.J.: Proceedings of Fifth Annual Contractor´s<br />
Meeting on Contaminant Contro in Hot Coal-Derived<br />
Gas Streams. U. S. Department of Energy/METC. Morgantown,<br />
WV. DOE/ METC-85/6025, pp. 6-18. Morgantown<br />
1985.<br />
3. Lamoreaux R.M., Brittain R.D., Zieger J., Leach. S.C.:<br />
Determination of Solid Phase Boundaries in Coal Gas<br />
Desulfurization by Zinc Ferrite., U. S. Department of<br />
Energy/METC. Technical Report DOE/MC 21096-219<strong>2.</strong><br />
Morgantown, WV, 1986.<br />
4. Sasaoka E., Hatori M., Sada n., Uddin A.: Ind. Eng.<br />
Chem. Res. 39, 3844 (2000).<br />
5. Buchanan R. E. and Bigons n. E. Bergy´s Manual of<br />
Determinative Bacteriology., 8 th ed., Baltimore, 1974.<br />
6. Wutzer R., Steinike U., Lorenz P., Rossahl,B.: Brennstoff-Wärme-Kraft<br />
45, 477 (1993).<br />
7. Šepelák V., Rogachev Yu., Steinike U., Uecker D. Ch.,<br />
Krumeich F., Wissmann S., Becker K. D.: Brennstoff-<br />
Wärme-Kraft 48, 1996, 28.<br />
8. Barret J., Hughes M. n., Karavajko G. I., Spencer P. A.:<br />
Metal extraction by bacterial oxidation of minerals. Eôis<br />
Horwood, new York, London, Toronto, Sydney, Tokyo,<br />
Singapore, 1996.<br />
9. Baláž P., Kušnierová M., Varencova V. I., Mišura B.: J.<br />
Miner. Process. 40, 273 (1994).