PROTEASES FROM CELL CULTURE OF Bromelia hemisphaerica ...
PROTEASES FROM CELL CULTURE OF Bromelia hemisphaerica ...
PROTEASES FROM CELL CULTURE OF Bromelia hemisphaerica ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
FSB1 – 2004<br />
Food Science and Biotechnology in Developing Countries<br />
V (U cm -3 )<br />
6<br />
5<br />
4<br />
3<br />
2<br />
1<br />
0<br />
Free<br />
0.0 0.5 1.0 1.5<br />
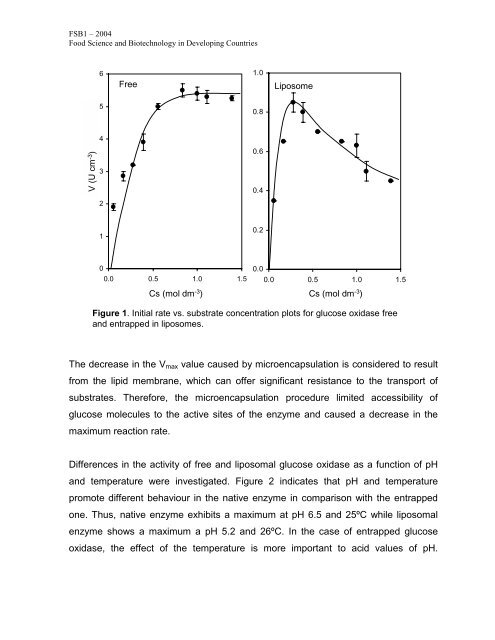
Figure 1. Initial rate vs. substrate concentration plots for glucose oxidase free<br />
and entrapped in liposomes.<br />
The decrease in the Vmax value caused by microencapsulation is considered to result<br />
from the lipid membrane, which can offer significant resistance to the transport of<br />
substrates. Therefore, the microencapsulation procedure limited accessibility of<br />
glucose molecules to the active sites of the enzyme and caused a decrease in the<br />
maximum reaction rate.<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0.0<br />
Liposome<br />
0.0 0.5 1.0 1.5<br />
Cs (mol dm -3 ) Cs (mol dm -3 )<br />
Differences in the activity of free and liposomal glucose oxidase as a function of pH<br />
and temperature were investigated. Figure 2 indicates that pH and temperature<br />
promote different behaviour in the native enzyme in comparison with the entrapped<br />
one. Thus, native enzyme exhibits a maximum at pH 6.5 and 25ºC while liposomal<br />
enzyme shows a maximum a pH 5.2 and 26ºC. In the case of entrapped glucose<br />
oxidase, the effect of the temperature is more important to acid values of pH.