Mesoscopic models of lipid bilayers and bilayers with embedded ...
Mesoscopic models of lipid bilayers and bilayers with embedded ... Mesoscopic models of lipid bilayers and bilayers with embedded ...
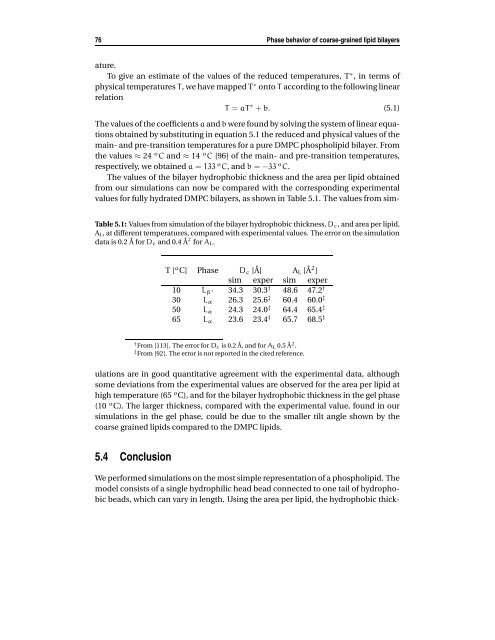
76 Phase behavior of coarse-grained lipid bilayers ature. To give an estimate of the values of the reduced temperatures, T ∗ , in terms of physical temperatures T, we have mapped T ∗ onto T according to the following linear relation T = aT ∗ + b. (5.1) The values of the coefficients a and b were found by solving the system of linear equations obtained by substituting in equation 5.1 the reduced and physical values of the main- and pre-transition temperatures for a pure DMPC phospholipid bilayer. From the values ≈ 24 o C and ≈ 14 o C [96] of the main- and pre-transition temperatures, respectively, we obtained a = 133 o C, and b = −33 o C. The values of the bilayer hydrophobic thickness and the area per lipid obtained from our simulations can now be compared with the corresponding experimental values for fully hydrated DMPC bilayers, as shown in Table 5.1. The values from sim- Table 5.1: Values from simulation of the bilayer hydrophobic thickness, Dc, and area per lipid, AL, at different temperatures, compared with experimental values. The error on the simulation data is 0.2 ˚A for Dc and 0.4 ˚A 2 for AL. T [ o C] Phase Dc [˚A] AL [˚A 2 ] sim exper sim exper 10 Lβ ′ 34.3 30.3† 48.6 47.2 † 30 Lα 26.3 25.6 ‡ 60.4 60.0 ‡ 50 Lα 24.3 24.0 ‡ 64.4 65.4 ‡ 65 Lα 23.6 23.4 ‡ 65.7 68.5 ‡ † From [113]. The error for Dc is 0.2 ˚A, and for AL 0.5 ˚A 2 . ‡ From [92]. The error is not reported in the cited reference. ulations are in good quantitative agreement with the experimental data, although some deviations from the experimental values are observed for the area per lipid at high temperature (65 o C), and for the bilayer hydrophobic thickness in the gel phase (10 o C). The larger thickness, compared with the experimental value, found in our simulations in the gel phase, could be due to the smaller tilt angle shown by the coarse grained lipids compared to the DMPC lipids. 5.4 Conclusion We performed simulations on the most simple representation of a phospholipid. The model consists of a single hydrophilic head bead connected to one tail of hydrophobic beads, which can vary in length. Using the area per lipid, the hydrophobic thick-
5.4 Conclusion 77 ness, the order parameter, and the extent of chain overlap, we were able to characterize various bilayer phases. The simulations showed that different stable phases are obtained for a wide range of temperatures. We characterized the low temperature phase as a gel phase, and we reproduced the main order/disorder phase transition from a gel to a liquid crystalline phase. This transition temperature to the Lα phase increases with increasing tail length, as was also found experimentally. In bilayers consisting of single-tail lipids, only the non-interdigitated Lβ phase and the fluid Lα phase are observed. However, experiments show that if chaotropic salts are added to the system, the distance between the headgroups is increased, stabilizing the Lα phase. We show that at high enough head-head repulsion the Lα phase is indeed stabilized and that the low temperature phase is the interdigitated LβI phase. This suggests that it is possible to induce an interdigitated phase in bilayers of single-tail lipids by adding chaotropic salts to the system. From our results, we can conclude that a model consisting of a head bead connected to a single tail does not describe the phase behavior of a double-tail lipid correctly. Single-tail lipids spontaneously form a low temperature interdigitated phase for high enough values of the repulsion parameter between headgroups, while experimentally this phase is observed in bilayers consisting of double-tail lipids, but has to be induced. By lowering the value of the head-head repulsion the low temperature phase is the Lβ phase. In this phase the chains of single-tails lipids are ordered parallel to the bilayer normal, while for most common double-tail phospholipids the hydrocarbon tails show a tilt with respect to the bilayer normal (the Lβ ′). We have hence increased the topological complexity of the model by considering model lipids with two hydrophobic tails. We have shown that the bilayers formed by these lipids display a phase behavior as function of temperature that well reproduces the phase behavior of phosphatidylcholine bilayers. Moreover, by defining a coarse-graining level in which a DPD bead corresponds to a volume of 90 ˚A 3 , i.e. to three water molecules or three methyl groups, we have defined a correspondence between a coarse-grained lipid with three head-beads and two tails of five beads each and the DMPC phospholipid. By also mapping the model reduced temperature, via the phase transition temperatures of DMPC bilayers, onto physical units, we were able to compare the simulated bilayer structural properties with their corresponding experimental values. A good agreement in the trends and values was found. This agreement validates our mesoscopic model for lipid bilayers.
- Page 31 and 32: 3.2 Method of calculation of surfac
- Page 33 and 34: 3.3 Constant surface tension ensemb
- Page 35 and 36: 3.3 Constant surface tension ensemb
- Page 37 and 38: 3.4 Surface tension in lipid bilaye
- Page 39 and 40: 3.4 Surface tension in lipid bilaye
- Page 41 and 42: 3.4 Surface tension in lipid bilaye
- Page 43 and 44: IV Structural characterization of l
- Page 45 and 46: 4.2 Structural quantities 39 been r
- Page 47 and 48: 4.3 Computational details 41 lipid
- Page 49 and 50: 4.4 Results and discussion 43 ρ(z)
- Page 51 and 52: 4.4 Results and discussion 45 one l
- Page 53 and 54: 4.4 Results and discussion 47 WH HT
- Page 55 and 56: 4.4 Results and discussion 49 Shill
- Page 57 and 58: 4.4 Results and discussion 51 chain
- Page 59 and 60: 4.4 Results and discussion 53 4.4.4
- Page 61: 4.4 Results and discussion 55 headg
- Page 64 and 65: 58 Phase behavior of coarse-grained
- Page 66 and 67: 60 Phase behavior of coarse-grained
- Page 68 and 69: 62 Phase behavior of coarse-grained
- Page 70 and 71: 64 Phase behavior of coarse-grained
- Page 72 and 73: 66 Phase behavior of coarse-grained
- Page 74 and 75: 68 Phase behavior of coarse-grained
- Page 76 and 77: 70 Phase behavior of coarse-grained
- Page 78 and 79: 72 Phase behavior of coarse-grained
- Page 80 and 81: 74 Phase behavior of coarse-grained
- Page 85 and 86: VI Interaction of small molecules w
- Page 87 and 88: 6.2 Computational details 81 For re
- Page 89 and 90: 6.3 Results and Discussion 83 withi
- Page 91 and 92: 6.3 Results and Discussion 85 ρ(z)
- Page 93 and 94: 6.3 Results and Discussion 87 ρ(z)
- Page 95 and 96: 6.3 Results and Discussion 89 S m 0
- Page 97 and 98: 6.3 Results and Discussion 91 π(z)
- Page 99: 6.3 Results and Discussion 93 the l
- Page 102 and 103: 96 Mesoscopic model for lipid bilay
- Page 104 and 105: 98 Mesoscopic model for lipid bilay
- Page 106 and 107: 100 Mesoscopic model for lipid bila
- Page 108 and 109: 102 Mesoscopic model for lipid bila
- Page 110 and 111: 104 Mesoscopic model for lipid bila
- Page 112 and 113: 106 Mesoscopic model for lipid bila
- Page 114 and 115: 108 Mesoscopic model for lipid bila
- Page 116 and 117: 110 Mesoscopic model for lipid bila
- Page 118 and 119: 112 Mesoscopic model for lipid bila
- Page 120 and 121: 114 Mesoscopic model for lipid bila
- Page 122 and 123: 116 Mesoscopic model for lipid bila
- Page 125 and 126: References [1] Tanford, C. Science
- Page 127 and 128: 7.4 Conclusion 121 [68] Ono, S.; Ko
- Page 129 and 130: 7.4 Conclusion 123 [139] Lee, A. Bi
- Page 131 and 132: Summary Biological membranes, as th
76 Phase behavior <strong>of</strong> coarse-grained <strong>lipid</strong> <strong>bilayers</strong><br />
ature.<br />
To give an estimate <strong>of</strong> the values <strong>of</strong> the reduced temperatures, T ∗ , in terms <strong>of</strong><br />
physical temperatures T, we have mapped T ∗ onto T according to the following linear<br />
relation<br />
T = aT ∗ + b. (5.1)<br />
The values <strong>of</strong> the coefficients a <strong>and</strong> b were found by solving the system <strong>of</strong> linear equations<br />
obtained by substituting in equation 5.1 the reduced <strong>and</strong> physical values <strong>of</strong> the<br />
main- <strong>and</strong> pre-transition temperatures for a pure DMPC phospho<strong>lipid</strong> bilayer. From<br />
the values ≈ 24 o C <strong>and</strong> ≈ 14 o C [96] <strong>of</strong> the main- <strong>and</strong> pre-transition temperatures,<br />
respectively, we obtained a = 133 o C, <strong>and</strong> b = −33 o C.<br />
The values <strong>of</strong> the bilayer hydrophobic thickness <strong>and</strong> the area per <strong>lipid</strong> obtained<br />
from our simulations can now be compared <strong>with</strong> the corresponding experimental<br />
values for fully hydrated DMPC <strong>bilayers</strong>, as shown in Table 5.1. The values from sim-<br />
Table 5.1: Values from simulation <strong>of</strong> the bilayer hydrophobic thickness, Dc, <strong>and</strong> area per <strong>lipid</strong>,<br />
AL, at different temperatures, compared <strong>with</strong> experimental values. The error on the simulation<br />
data is 0.2 ˚A for Dc <strong>and</strong> 0.4 ˚A 2 for AL.<br />
T [ o C] Phase Dc [˚A] AL [˚A 2 ]<br />
sim exper sim exper<br />
10 Lβ ′ 34.3 30.3† 48.6 47.2 †<br />
30 Lα 26.3 25.6 ‡ 60.4 60.0 ‡<br />
50 Lα 24.3 24.0 ‡ 64.4 65.4 ‡<br />
65 Lα 23.6 23.4 ‡ 65.7 68.5 ‡<br />
† From [113]. The error for Dc is 0.2 ˚A, <strong>and</strong> for AL 0.5 ˚A 2 .<br />
‡ From [92]. The error is not reported in the cited reference.<br />
ulations are in good quantitative agreement <strong>with</strong> the experimental data, although<br />
some deviations from the experimental values are observed for the area per <strong>lipid</strong> at<br />
high temperature (65 o C), <strong>and</strong> for the bilayer hydrophobic thickness in the gel phase<br />
(10 o C). The larger thickness, compared <strong>with</strong> the experimental value, found in our<br />
simulations in the gel phase, could be due to the smaller tilt angle shown by the<br />
coarse grained <strong>lipid</strong>s compared to the DMPC <strong>lipid</strong>s.<br />
5.4 Conclusion<br />
We performed simulations on the most simple representation <strong>of</strong> a phospho<strong>lipid</strong>. The<br />
model consists <strong>of</strong> a single hydrophilic head bead connected to one tail <strong>of</strong> hydrophobic<br />
beads, which can vary in length. Using the area per <strong>lipid</strong>, the hydrophobic thick-