EFFECTS OF ACID RAIN
EFFECTS OF ACID RAIN
EFFECTS OF ACID RAIN
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>EFFECTS</strong> <strong>OF</strong> <strong>ACID</strong> <strong>RAIN</strong><br />
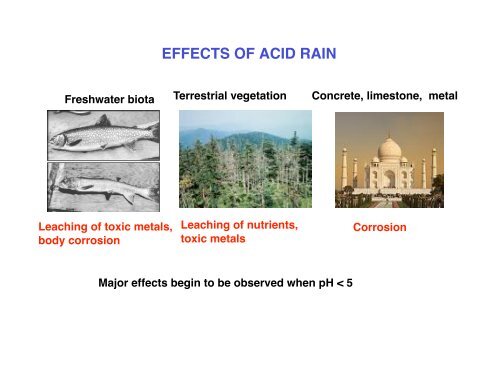
Freshwater biota Terrestrial vegetation Concrete, limestone, metal<br />
Leaching of toxic metals,<br />
body corrosion<br />
Leaching of nutrients,<br />
toxic metals<br />
Major effects begin to be observed when pH < 5<br />
Corrosion
NATURAL pH <strong>OF</strong> <strong>RAIN</strong><br />
• Equilibrium with natural CO 2 (280 ppmv) results in a rain pH of 5.7:<br />
• This pH can be modified by natural acids (H 2 SO 4 , HNO 3 , RCOOH…)<br />
and bases (NH 3 , CaCO 3 ) natural rain has a pH in range 5-7<br />
“Acid rain” refers to rain with pH < 5 damage to ecosystems
PRECIPITATION PH OVER THE UNITED STATES
Graedel and Crutzen, Atmospheric<br />
Change (1992)<br />
<strong>ACID</strong> FOG<br />
Like acid rain but more concentrated;<br />
pH values as low as 2.<br />
Effects on terrestrial ecosystems, structures<br />
thatʼs me!
CHEMICAL COMPOSITION <strong>OF</strong> PRECIPITATION
Chemical reactions leading to acidity in rain<br />
Gas phase oxidation of SO 2 ( 1 – 2 weeks)<br />
SO 2 + OH + M HSO 3 + M<br />
HSO 3 + O 2 SO 3 + HO 2<br />
SO 3 + H 2O H 2SO 4<br />
Liquid phase (in cloud) oxidation of SO 2 ( ~ days )<br />
SO 2 SO 2 H 2 O HSO 3 - + H +<br />
(add H 2 O 2 from atm)<br />
HSO 3 - + H2O 2 + H + SO 4 = + 2H + + H2O<br />
6
Neutralization of acidity in rainwater by NH 3 is illusory because in the<br />
ecosystem/soils<br />
NH 4 + + 3/2 O2 NO 2 - + 2H + + H2 O<br />
NO 2 - + ½ O2 NO 3 - (nitrification) in a system with lots of N<br />
If taken up and turned into organic matter, effectively<br />
NH 4 + "" NH3 + H +<br />
Neutralization of acidity in rainwater by base cations from soil is effective.<br />
CaCO 3 Ca +2 + CO 3 -2<br />
CO 3 -2 + H + HCO3 -<br />
7
LONG-TERM TREND IN US SO 2 EMISSIONS
AMMONIUM AND SULFATE TRENDS, 1985-2004<br />
Lehmann et al. [2007]<br />
NH 4 +<br />
SO 4 2-
TREND IN FREQUENCY <strong>OF</strong> <strong>ACID</strong> <strong>RAIN</strong> (pH < 5)<br />
Lehmann et al. [2007]<br />
1994-1996<br />
2002-2004
BUT ECOSYSTEM <strong>ACID</strong>IFICATION IS PARTLY A TITRATION PROBLEM<br />
FROM <strong>ACID</strong> INPUT OVER MANY YEARS<br />
Acid flux<br />
F H+<br />
Acid-neutralizing capacity (ANC)<br />
from CaCO 3 and other bases
NO 3 -<br />
pH<br />
precipitation<br />
SO 4 -2<br />
stream water<br />
SO 4 -2
DEPLETION <strong>OF</strong> BASE CATIONS FROM <strong>ACID</strong> <strong>RAIN</strong> <br />
(Hubbard Brook Experimental Forest, New Hampshire)
stream<br />
precip<br />
15
Acid precipitation is caused by emissions of SO 2 and<br />
NO x, which are converted to mineral acids in the<br />
atmosphere.<br />
The influx of acidic precipitation can damage<br />
ecosystems by disrupting nutrient cycles, availability of<br />
base cations (Ca +2 , K + ) to plants, and by direct damage<br />
to plant organs (mostly acid fog).<br />
Cap-and-trade legislation has been very effective in<br />
reducing acid deposition but emissions, and lasting<br />
ecological effects from the past, remain.<br />
16