- Page 1 and 2:
MODELING CHAR OXIDATION AS A FUNCTI
- Page 3 and 4:
BRIGHAM YOUNG UNIVERSITY As chair o
- Page 5 and 6:
CBK model uses: 1) an intrinsic Lan
- Page 7 and 8:
Table of Contents List of Figures..
- Page 9:
Appendices.........................
- Page 12 and 13:
Figure A.2. Mass releases of the Ko
- Page 14 and 15:
Table 7.6. Parameters Used in Model
- Page 16 and 17:
Ed activation energy of desorption,
- Page 18 and 19: vc the volume of combustible materi
- Page 21 and 22: Background 1. Introduction The rate
- Page 23: the CBK model developed at Brown Un
- Page 26 and 27: Zone III rate ∝ C og E obs → 0
- Page 28 and 29: coal-general kinetic rate constants
- Page 30 and 31: Boundary Layer Diffusion The molar
- Page 32 and 33: = q obs q max The factor can be use
- Page 34 and 35: where k 1 and K are two kinetic par
- Page 36 and 37: particle can therefore be convenien
- Page 38 and 39: This is the first time that the gen
- Page 40 and 41: Data of Mathias Mathias (1996) perf
- Page 42 and 43: urn with shrinking diameters, and t
- Page 45 and 46: 3. Objectives and Approach The obje
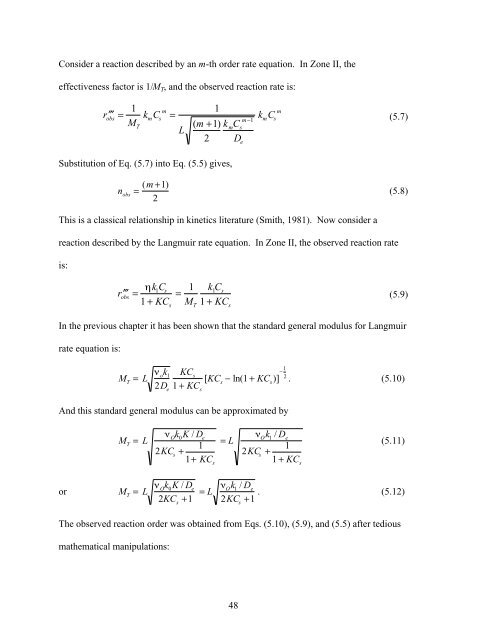
- Page 47 and 48: Introduction 4. Analytical Solution
- Page 49 and 50: Task and Methodology Task One of th
- Page 51 and 52: 2 [ (i +1) − (i − 1)] i b = −
- Page 53 and 54: Table 4.1. The Relative Error * (%)
- Page 55 and 56: The resulting observations regardin
- Page 57 and 58: correction. The values of functions
- Page 59 and 60: Table 4.6. The Relative Error* (%)
- Page 61 and 62: Table 4.8. The Relative Error* (%)
- Page 63 and 64: general asymptotic solution. An arc
- Page 65 and 66: 5. Theoretical Developments The int
- Page 67: order of a reaction is usually dete
- Page 71 and 72: The observed reaction order in Zone
- Page 73 and 74: Bulk Diffusion vs. Knudsen Diffusio
- Page 75 and 76: where D K is in cm 2 /sec, r p is t
- Page 77 and 78: where T p is in K, P is in atm. The
- Page 79 and 80: Both of these assumptions are argua
- Page 81 and 82: 2 r obs ′ − [kD Pog + k d + kD
- Page 83 and 84: oxygen partial pressure (Suuberg et
- Page 85 and 86: Farrauto and Batholomew (1997) prop
- Page 87: assumes a homogeneous, non-interact
- Page 90 and 91: Single-Film Char Oxidation Submodel
- Page 92 and 93: where and An energy balance is used
- Page 94 and 95: where is the empirical burning mode
- Page 96 and 97: calculation uses a 7 × 7 × 7 matr
- Page 98 and 99: HP-CBK Model Development The HP-CBK
- Page 100 and 101: Effective Diffusivity The major obs
- Page 102 and 103: where r p1 and r p2 are the average
- Page 104 and 105: where r p1 is the macro-pore radius
- Page 107 and 108: 7. Model Evaluation and Discussion
- Page 109 and 110: experiments are non-porous, the rat
- Page 111 and 112: and 2850 K). For consistency with t
- Page 113 and 114: The value of the roughness factor w
- Page 115 and 116: = S int S ext D e r p a 2 2M C M O2
- Page 117 and 118: Reactor Head Flow Straightener Reac
- Page 119 and 120:
the large size of the particle, and
- Page 121 and 122:
taking into account convection, rad
- Page 123 and 124:
2.5x10 -4 2 /sec) 2.0 1.5 Rate (g/c
- Page 125 and 126:
Table 7.5. The Experimental Conditi
- Page 127 and 128:
The burnout and particle velocity d
- Page 129 and 130:
The HP-CBK was used to predict the
- Page 131 and 132:
TGA and FFB Data-This Study The rea
- Page 133 and 134:
This equation can be derived as fol
- Page 135 and 136:
q = A 1p e − E 1 p / RT P os 1 +
- Page 137 and 138:
m obs = 0 at high temperatures) and
- Page 139 and 140:
Currently the correlations between
- Page 141 and 142:
8. Summary and Conclusions The obje
- Page 143 and 144:
0.5 due to the contribution from th
- Page 145 and 146:
Langmuir rate equation, the reactio
- Page 147 and 148:
II, in agreement with many observat
- Page 149 and 150:
9. Recommendations The predictive c
- Page 151 and 152:
References Ahmed, S., M. H. Back an
- Page 153 and 154:
Essenhigh, R. H., D. Fortsch and H.
- Page 155 and 156:
Mehta, B. N. and R. Aris , “Commu
- Page 157 and 158:
Szekely, J. and M. Propster, "A Str
- Page 159 and 160:
Appendices 139
- Page 161 and 162:
Introduction Appendix A: Experiment
- Page 163 and 164:
detaching the flame from the burner
- Page 165 and 166:
To study the effects of steam, CO w
- Page 167 and 168:
times at heights of 1, 2, 4, and 6
- Page 169 and 170:
analysis. The char reactivities (in
- Page 171 and 172:
Table A.5. Moisture, Ash and ICP Ma
- Page 173 and 174:
Table A.9. Elemental Analyses of Fo
- Page 175 and 176:
temperature profile of the post-fla
- Page 177 and 178:
Apparent densities 1.00 0.75 0.50 0
- Page 179 and 180:
This observation is somewhat surpri
- Page 181 and 182:
It is interesting to compare Figure
- Page 183 and 184:
The N 2 BET surfacea areas and H/C
- Page 185 and 186:
collected in the #4 reactor conditi
- Page 187 and 188:
Rate (gC /g C remaining /sec) 1.6x1
- Page 189 and 190:
close to zero, the accumulated erro
- Page 191:
Appendix B: Errors and Standard Dev