Integrated Cell Processing Work Station — CPWS - Sanyo
Integrated Cell Processing Work Station — CPWS - Sanyo
Integrated Cell Processing Work Station — CPWS - Sanyo
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>CPWS</strong> Features:<br />
www.sanyobiomedical.com<br />
My Life. My <strong>Work</strong>. My Choice.<br />
<strong>Integrated</strong> <strong>Cell</strong><br />
<strong>Processing</strong> <strong>Work</strong><br />
<strong>Station</strong> <strong>—</strong> <strong>CPWS</strong><br />
PPLF-4205<br />
• Cost effective, space-saving solution<br />
for GMP and GTP compliant regenerative<br />
medicine and cell therapy.<br />
• Minimizes the expense of a cleanroom laboratory
PPLF-4205<br />
SANYO <strong>CPWS</strong> <strong>Integrated</strong> <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong><br />
Minimizes Cleanroom Expense<br />
Self-Contained<br />
Space-Saving<br />
Quick to Acquire and Install<br />
GMP and FDA Compliant for Aseptic Process<br />
User Friendly<br />
Energy Efficient, Green Design<br />
The Essential <strong>Work</strong> <strong>Station</strong> for <strong>Cell</strong> Therapeutics<br />
Continued worldwide development of new methods and processes in cell therapy and<br />
regenerative medicine requires renewed emphasis on tools and technologies required to<br />
establish and maintain aseptic conditions demanded of the clinical environment. SANYO<br />
has met that challenge.<br />
2 CWPS <strong>Integrated</strong> <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong> www.sanyobiomedical.com<br />
In designing the industry’s first viable alternative to a conventional Class 10,000 cleanroom<br />
for both Class 100 air quality and barrier isolation, SANYO has introduced the <strong>Cell</strong> <strong>Processing</strong><br />
<strong>Work</strong> <strong>Station</strong> (<strong>CPWS</strong>) to bring the potential of cell therapeutics to more facilities while<br />
mitigating acquisition and operation costs and protracted time lines.<br />
Ophthalmalogy and<br />
Organ Replacement/<br />
Preservation<br />
Dental/Oral<br />
Skin<br />
Urology<br />
Global <strong>Cell</strong> Therapy and<br />
Tissue Engineering Market<br />
2010 & 2015<br />
General<br />
Cancer<br />
Cord Blood and <strong>Cell</strong> Banking<br />
Orthopedics/Spine<br />
Cardiology<br />
Neurology<br />
Millions of Dollars<br />
$25,000<br />
$20,000<br />
$15,000<br />
$10,000<br />
$5,000<br />
$0<br />
2010 2015<br />
<strong>Cell</strong>/Tissue<br />
Banking<br />
Cancer<br />
General Surgery,<br />
GI, Gyn, Others<br />
Ophthalmalogy<br />
Organ<br />
Dental<br />
Skin<br />
Urology<br />
Ortho<br />
Neuro<br />
Cardio
The Market<br />
Immediate beneficiaries of the SANYO<br />
<strong>CPWS</strong> include both private and public<br />
institutions serving the life science,<br />
clinical, biotechnology and pharmaceutical<br />
markets. These include mainstream<br />
and scale-up pharmaceutical companies<br />
focused on drug and medical device<br />
development related to human cells,<br />
medical researchers applying cellular<br />
therapies in clinical trials, and hospitals<br />
now deploying FDA approved cellular<br />
therapeutics to treat a broad range<br />
of diseases.<br />
As the science progresses and results<br />
are propagated throughout the medical<br />
and scientific community, the need for<br />
bench-level research and production is<br />
expanding exponentially. As a result, the<br />
availability of the SANYO free-standing,<br />
self-contained work station apart from<br />
the conventional layered cleanroom<br />
approach to containment and protection,<br />
is bringing the capability for on-site<br />
cellular therapeutics to more facilities<br />
more quickly and at a lower capital and<br />
operational cost, all within the GMP<br />
performance envelope required of the<br />
cell technology itself.<br />
Self-Contained System,<br />
Small Footprint<br />
The SANYO concept of a self-contained<br />
work station is enabled by the company’s<br />
demonstrated proficiency for in situ<br />
decontamination required to separate<br />
processes from one patient to the next,<br />
or one protocol to another. By using a<br />
highly effective H2O2 decontamination<br />
process, the <strong>CPWS</strong> can be completely<br />
sterilized without heat and prepared for<br />
the next protocol within two hours as<br />
compared to a cleanroom decontamination<br />
function can take days or weeks.<br />
By increasing throughput within the<br />
parameters of GMP compliance and FDA<br />
guidelines, the <strong>CPWS</strong> is deployed for<br />
both<br />
research, cellular manipulation and<br />
growth, cell product extraction and<br />
emerging processes that fall within<br />
similar guidelines.<br />
Current and future applications of the<br />
<strong>CPWS</strong> include organ and tissue regeneration<br />
such as skin, cartilage, alveolar bone,<br />
cornea, cardiac muscle, nerve, liver and<br />
pancreas regeneration. Immunotherapy<br />
applications may extend to dendritic<br />
cells, T-cells and more.<br />
Making it <strong>Work</strong><br />
Improving efficiency in human interaction<br />
is a primary design attribute of the<br />
<strong>CPWS</strong>. All work must be performed without<br />
human error, in an aseptic environment,<br />
and with detailed documentation<br />
to assure quality and compliance. Gownup<br />
time, expense and inconvenience is<br />
minimized or eliminated altogether. <strong>Integrated</strong><br />
systems within the <strong>CPWS</strong> permit<br />
cellular extraction, preparation, culturing<br />
and administration with aseptic assurance<br />
as well as economic practicality.<br />
Because conventional autoclaving is<br />
not possible, the SANYO H2O2 decontamination<br />
process diminishes both time<br />
and labor associated with this critical<br />
step between patients, positioning the<br />
<strong>CPWS</strong> squarely in the equation for cost/<br />
benefit justification associated with<br />
investment decision-making. Allowances<br />
for integrated centrifuge, microscopy,<br />
data acquisition and incubation functions<br />
are important considerations in the<br />
<strong>CPWS</strong> design; a unique docking station<br />
permits interface and exchange with<br />
unlimited number of cell culture incubators<br />
dedicated to individual patients or<br />
cell lines.<br />
On the <strong>Work</strong> Surface<br />
Since humans remain the single most<br />
common source of contamination, the<br />
<strong>CPWS</strong> provides both physical and process<br />
benefits to minimize contamination<br />
and cross-contamination in the work<br />
area. Within the four-port glove box the<br />
<strong>CPWS</strong> delivers more than a conventional<br />
Class II, Type A2 biological safety cabinet<br />
typically installed within a cleanroom to<br />
achieve the same objective. Here, the<br />
manually initiated, automatically deployed<br />
H2O2 decontamination process supplements<br />
continuous HEPA filtration. As<br />
0.3 micron particles are removed from<br />
the fresh air exchange, H2O2 decontamination<br />
neutralizes contaminants brought<br />
forth by instrumentation or equipment.<br />
A number of sterilization sequences are<br />
available to protect the aseptic environ-<br />
ment.<br />
The glove box design offers barrier isolation<br />
protection for the operator and the<br />
work inside. User comfort and ergonomics<br />
are inherent to the <strong>CPWS</strong> design,<br />
including a sloped front for easier access<br />
and glare reduction.<br />
What You Don’t Need<br />
The relatively small footprint permits<br />
installation into existing or new Class<br />
100,000 lab space with conventional<br />
utilities and minimal site preparation.<br />
Multi-layered airlocks in multiple treated<br />
rooms are avoided. Capital intensive<br />
expenses are lowered and lead times<br />
from decision to operation are shortened.<br />
Once in place, operational costs are<br />
highly contained and predictable, with<br />
sterilization available more frequently and<br />
at a fraction of conventional cost.<br />
The SANYO Difference<br />
SANYO Biomedical, a division of SANYO<br />
North American Corporation, is headquartered<br />
in suburban Chicago. For over<br />
forty years, SANYO has established a<br />
reputation as a premier manufacturer of<br />
precision biomedical and laboratory equipment.<br />
Known throughout the world as<br />
a leading brand in consumer electronics<br />
and appliances, SANYO addresses global<br />
needs such as energy, food, housing,<br />
healthcare and information technology.<br />
As a part of the SANYO product line<br />
worldwide, the <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong><br />
<strong>Station</strong> exemplifies our unique Vertical<br />
Component Integration approach to<br />
product development, combining ideas<br />
and innovations from our global industrial<br />
and consumer products network into an<br />
integrated product featuring advanced<br />
technology, controls, construction and<br />
performance attributes.<br />
The <strong>CPWS</strong> and subcomponent systems<br />
have been extensively tested to meet the<br />
toughest quality standards for performance,<br />
ergonomics and cost of ownership.<br />
The <strong>CPWS</strong> is designed to minimize<br />
its carbon footprint through energy savings<br />
and environmental stewardship.<br />
3
SANYO <strong>CPWS</strong> Series Technical Attributes<br />
The SANYO <strong>CPWS</strong> work station is<br />
designed to deliver efficient, costeffective<br />
and GMP compliant cell<br />
therapy and manufacturing capability<br />
without the expense and inconvenience<br />
of a class 10,000 cleanroom. The<br />
<strong>CPWS</strong> offers significant advantages<br />
over conventional hard wall cleanroom<br />
construction.<br />
• The <strong>CPWS</strong> is less expensive<br />
than a cleanroom.<br />
• It is quicker to acquire and place<br />
into operation.<br />
• The small footprint increases options<br />
for location and orientation.<br />
• The user-friendly glove box design<br />
eliminates gowning and improves<br />
operator comfort and convenience.<br />
• Operating costs are lower than<br />
cleanroom costs<br />
• <strong>Work</strong> is easily suspended and<br />
resumed without the need to<br />
de-gown and re-gown, improving<br />
user comfort.<br />
• Fast decontamination and changeover<br />
improve productivity, increase<br />
throughput and deliver quicker return<br />
on investment.<br />
• Recordkeeping and process<br />
documentation are easier to manage.<br />
Components and operating systems are<br />
configured around a central work station<br />
with a HEPA filtration and air management<br />
system designed to deliver Class<br />
100 air to the work surface within the<br />
glove box.<br />
• Central barrier isolator<br />
• Pass box interchange<br />
• <strong>Integrated</strong> H2O2<br />
decontamination<br />
system<br />
• Optional cell observation system with<br />
microscope and monitor<br />
• Optional centrifuge integrated into<br />
the work surface<br />
• Optional CO2<br />
incubator with<br />
docking collar<br />
• The optional incubator and optional<br />
centrifuge operate within a Class 100<br />
environment.<br />
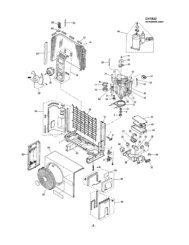
<strong>CPWS</strong> <strong>Work</strong> <strong>Station</strong><br />
The SANYO <strong>CPWS</strong> work station is a<br />
component-based design that permits<br />
long-term or quick turnover selfcontained<br />
protocols with efficiency and<br />
safety for the product as well as personnel.<br />
As an integrated system, all functions<br />
associated with good laboratory<br />
technique, environmental control and<br />
ergonomic comfort are selected for compatibility<br />
and complementary functional<br />
performance.<br />
1. Modular CO2 incubator, shown on<br />
cart, docked to barrier isolator.<br />
2. Incubator cart with locking casters;<br />
offset casters nest with<br />
frame assembly when docked.<br />
3. Lid cam latch<br />
4. Centrifuge controller<br />
5. Centrifuge module accessible<br />
for the aseptic work area.<br />
6. Glove port<br />
4 CWPS <strong>Integrated</strong> <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong> www.sanyobiomedical.com<br />
1<br />
2<br />
14<br />
15<br />
18<br />
3<br />
7. Hinged front access assembly;<br />
front lifts up when total interior<br />
access is required.<br />
8. Interchange pass box with manually<br />
initiated, automatic sequence H2O2<br />
decontamination system.<br />
9. System controller<br />
10. H2O2 liquid supply cartridge<br />
11. HEPA supply and exhaust filtration<br />
blower motor assembly<br />
12. Electrical compartment<br />
13. Interior fluorescent lamps<br />
14. Electropolished interior surfaces<br />
15. Angled 6° front for user comfort,<br />
reduced glare<br />
16. Locking screws to secure<br />
hinged front.<br />
17. Adjustable leveling feet<br />
18. Optional cell monitor LCD<br />
7<br />
5<br />
16<br />
11<br />
17<br />
6<br />
13<br />
4<br />
8<br />
9<br />
12<br />
10
SANYO <strong>CPWS</strong> Series Applications<br />
Clinical Applications of <strong>Cell</strong> Therapies<br />
Disease States <strong>Cell</strong> Therapies<br />
Cancer<br />
Hematopoietic Stem <strong>Cell</strong><br />
(HSC) Transplantation<br />
Immunotherapy<br />
Orthopedic<br />
Autologous and allogeneic HSC; Ex vivo expansion of HSC;<br />
‘Suicide’ T-cells – gene transfer; Stem cell transplantation<br />
Dendritic cells; NK/T cells; Macrophage-activated killer cells;<br />
T-cell expansion; NK cells; Co-stimulatory molecules (gene transfer)<br />
Expanded chondrocytes; Mesenchymal stem cells<br />
Neurodegenerative Disorders/Trauma<br />
Adult stem cell-derived cells; Embryonic stem cell-derived neural cells<br />
Cardiovascular Disease<br />
Infusion of marrow/blood-derived angio blasts;<br />
CD34 stem cells; cardiac cells<br />
Organ Replacement<br />
Pancreas (diabetes)<br />
Pancreatic islet cells; Embryonic stem cell-derived islet cells;<br />
Adult stem cell-derived islet cells<br />
Liver (failure, metabolic disorders) Bioartificial liver; Isolated hepatocytes; Hepatocyte stem cells<br />
Kidney (failure) Bioartificial kidney<br />
Wound Healing<br />
Infectious Diseases<br />
Keratinocytes; Skin stem cells<br />
Antigen-loaded dendritic cells; Lymphocyte expansion; Macrophages<br />
Genetic Deficiencies<br />
Hemophilia Gene Therapy<br />
SCID Gene Therapy<br />
Cystic Fibrosis Gene Therapy<br />
Autoimmune Diseases<br />
Immunotherapy<br />
Dendritic cells; T-cells, Mesenchymal stem cells;<br />
Lymphocyte expansion; Natural Killer cells<br />
HSC, hematopoietic stem cells; NK, Natural Killer; SCID, severe combined immunodeficiency<br />
Currently in clinical studies.<br />
Applications<br />
The SANYO <strong>CPWS</strong> enables a broader<br />
access to cell therapeutics related to<br />
both minimally manipulated and nonminimally<br />
manipulated cell products by<br />
lowering the cost of entry, extending<br />
the process to the widest range of<br />
applications, and minimizing operating<br />
expenses when compared to a conventional<br />
cleanroom environment.<br />
• Minimally manipulated products<br />
are associated with cell washing,<br />
enrichment, selection, HSC (PB,<br />
BM, CB), cancer therapies and<br />
other under GTP requirements.<br />
• Non-minimally manipulated<br />
products are associated with<br />
expanded, differentiated or<br />
transformed cells (DC, MSC, ESC,<br />
TC) in cancer centers, biotech labs,<br />
stem cell institutes and contract<br />
manufacturing facilities operating<br />
under GMP requirements.<br />
• GMPs (Good Manufacturing<br />
Practices) are mandated by the<br />
United States Food and Drug<br />
Administration to ensure that drug<br />
development and manufacturing<br />
is safe, quality controlled for<br />
repeatability and thoroughly<br />
documented.<br />
• GMPs typically require expensive<br />
hard wall laboratories and laboratory<br />
suites using biological safety<br />
cabinets in Class 10,000 cleanrooms<br />
surrounded by a Class 100,000 room.<br />
5
SANYO <strong>CPWS</strong> Series Benefits<br />
Comparative Operating Costs 1 (Nominal), SANYO <strong>CPWS</strong> vs. Conventional Cleanroom, Annual<br />
SANYO <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong> (<strong>CPWS</strong>) Conventional GMP Cleanroom<br />
Function Annual Budget<br />
(nominal)<br />
6 CWPS <strong>Integrated</strong> <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong> www.sanyobiomedical.com<br />
Function Annual Budget<br />
(nominal)<br />
H2O2 Cartridge, 25g, Total System Decontamination $9,000 Disinfection, Cleaning $9,000<br />
H2O2 Cartridge, 5g, Pass Box Only Decontamination $13,320 Routine Sanitation $12,000<br />
Residual H2O2 Detector $2,664 Particle Sensors $3,000<br />
Gloves, 3 gloves, 2× $1,140 Sterilized, Non-Shedding Gowns $18,000<br />
Sleeves, 3 gloves, 1× $3,640<br />
HEPA Filters HEPA Filters<br />
<strong>Work</strong> <strong>Station</strong> $907 Biosafety Cabinet, Supply Filter $343<br />
Interchange Pass Box $294 Biosafety Cabinet, Exhaust Filter $190<br />
Room $833 Certifier Labor, BSC Formalin Decontamination $956<br />
Labor $1,330 Cleanroom Air Supply $2,916<br />
Validation Expenses Validation Expenses<br />
Cleanroom Air Exhaust $1,516<br />
Certifier Labor, CR Formalin Decontamination $1,500<br />
Cleanliness and Air Volume Report $1,440 Biosafety Cabinet and Report $1,050<br />
Decontamination Performance BI Inspection $3,230 Cleanroom and Report $10,000<br />
H2O2 Mist Uniformity Inspection $720 Centrifuge and Report $1,350<br />
Centrifuge Module and Report $1,350 CO2 Incubator Module and Report $850<br />
CO2 Incubator Module and Report $1,250<br />
Approximately 35% Less Expensive to Operate<br />
The SANYO <strong>CPWS</strong> offers significantly lower operating costs when<br />
compared to a conventional cleanroom (open system). Comparison<br />
methodology and assumptions are available from SANYO. Softcost<br />
benefits related to user convenience and comfort, flexibility,<br />
throughput and other demonstrated advantages of the <strong>CPWS</strong> can be<br />
established through consultative review. Contact SANYO for details.<br />
Power Consumption $18,057 Power Consumption $28,972<br />
Nominal Annual Budget $60,100 Nominal Annual Budget $91,600<br />
1 Comparative operating costs listed herein are nominal and based on actual installations in Japan. Total cost of ownership and operation will vary according to type of<br />
installation, method of use, geographic location and other factors. SANYO offers consultative assistance in developing a cost/benefit analysis specific to your facility and<br />
protocol. Contact SANYO to arrange a report unique to your prospective application
SANYO <strong>CPWS</strong> Benefit vs. Conventional Cleanroom (Closed System vs. Open System)<br />
Comparison<br />
General<br />
SANYO <strong>CPWS</strong><br />
Barrier Isolator<br />
Closed system, requires only<br />
Class 100,000 air<br />
Lead Time and Cost Minimal; Cost of Class 100,000 room plus <strong>CPWS</strong><br />
Space/Footprint Allowance Minimal footprint in existing space<br />
Cleanroom<br />
with Biological Safety Cabinets<br />
Open system, requires significant investment<br />
and maintenance of background environment,<br />
Class 10,000 air<br />
High; Cost of Class 10,000 cleanroom plus Class 100<br />
one or more Class 100 biological safety cabinets<br />
Dedicated new/retrofit facility with significant<br />
requirement for HVAC, filtration, air showers<br />
Validation Costs Minimal; Requires Class 100,000 only. High; Requires both Class 100 and Class 100,000.<br />
Operation Cost Low<br />
High; Repeated decontamination and maintenance<br />
costs. Higher consumables cost<br />
Implementation Weeks Months or Years<br />
Ergonomics and User Comfort<br />
Flexibility<br />
Throughput<br />
No second gowning required. Central barrier isolator<br />
with ergonomically angled front permits easy access through<br />
glove ports. Reduces stress on workforce.<br />
Elimination of hazardous fumes for decontamination<br />
permits diverse applications. Elimination of depolymerizing<br />
formaldehyde, formalin and other toxic chemicals permits<br />
transition between applications seamlessly.<br />
Expanded. Quick changeover extends use,<br />
optimizes return on investment. Component integration<br />
streamlines workflow while enhancing aseptic processing.<br />
Conventional first and second gowning, external air<br />
supply, interlock doors/air showers<br />
Inflexible<br />
Limited due to product changeover criteria<br />
Security<br />
Decontamination and Sterilization Two Hours Up to Two Weeks<br />
Equipment and Instrumentation<br />
Microscopes and other instrumentation<br />
can be dedicated to the work station.<br />
Shared instrumentation in an open system exposes<br />
processes to cross contamination.<br />
Barrier Isolator<br />
The barrier isolator forms the central<br />
component to the work station and<br />
contains the primary operating systems<br />
required to establish and maintain aseptic<br />
conditions to meet GMP criteria.<br />
• The barrier completely isolates the interior<br />
work product from the operator.<br />
• The barrier isolator creates a closed<br />
Class 100 environment, eliminating<br />
the need for biological safety cabinets<br />
in a cleanroom.<br />
• Isolator interior air is 100% Class<br />
100 total circulation; no recirculated<br />
air is used.<br />
• The polished stainless steel glove<br />
box interior is designed for maximum<br />
exposure of all interior surfaces subject<br />
to H2O2 decontamination.<br />
• Before commissioning and initial use,<br />
the isolator front can be opened on<br />
a hinged frame for installation of instrumentation<br />
or other devices larger than<br />
the interchange pass box opening.<br />
• The HEPA filter and airflow system is<br />
mounted on top of the isolator.<br />
• The internal airflow system is designed<br />
to create a positive pressure to mitigate<br />
the possibility of inflow contamination.<br />
• Viability of the containment area<br />
is not delegated to third-party cleanroom<br />
contractors who are hired to<br />
decontaminate.<br />
Ergonomics and Safety<br />
Because second gowning is not required,<br />
user comfort and productivity is significantly<br />
improved. The workplace routine,<br />
including bathroom breaks, is unencumbered<br />
by the need to leave and re-enter<br />
a cleanroom, bleach and/or shower.<br />
• The inconvenience and expense of<br />
cumbersome containments suits with<br />
air and vacuum hoses is eliminated. If<br />
working with BL3 agents, the buddy<br />
system is not required.<br />
• The barrier eliminates the potential<br />
for room contamination from blood<br />
or other aerosols. <strong>Work</strong>flow is not<br />
impacted by routine colds.<br />
• Staff confidence in total containment<br />
improves morale.<br />
• Glove ports permit easier handling of<br />
red bag materials when required.<br />
• A 6º angled front includes three glove<br />
ports to permit access to all interior<br />
surfaces.<br />
• The interior cabinet includes independent<br />
interior fluorescent lamps to<br />
supplement ambient light.<br />
7
SANYO <strong>CPWS</strong> Series Features<br />
Aseptic Environment Required for <strong>Cell</strong> Preparation<br />
Usually, human derived cells must be guaranteed that they are prepared and cultured in an aseptic environment because<br />
they cannot be treated by heat or pressure.<br />
Original<br />
<strong>Cell</strong>s<br />
Standard of Particle Control<br />
ISO14644-1 FDA EU-GMP Annex 1 JP WHO-GMP TRS902 Annex 6<br />
Class 5<br />
Class 7<br />
Class 8<br />
Class 100<br />
In Operation 3,520<br />
Class 10,000<br />
In Operation 35,200<br />
Class 100,000<br />
In Operation 3,520,000<br />
Class 9 <strong>—</strong><br />
Extraction Preparation Culture Administration<br />
Grade A<br />
In Operation 3,500<br />
Grade B<br />
In Operation 350,000<br />
At rest 3,500<br />
Grade C<br />
In Operation 3,500,000<br />
At rest 350,000<br />
Grade D<br />
At rest 3,500,000<br />
H2O2 Decontamination System<br />
• The manually initiated, automatically<br />
sequenced H2O2 decontamination<br />
system offers a fast, safe and proven<br />
sterilization process to enhance<br />
the performance of the <strong>CPWS</strong> by<br />
allowing more frequent turnover of<br />
segregated cell lines.<br />
• The validated H2O2<br />
system generates<br />
an H2O2 vapor that permeates all<br />
exposed surfaces from the central<br />
interchange pass box nebulizer<br />
containing a replaceable bottle of<br />
enriched hydrogen peroxide.<br />
• When deployed, the H2O2<br />
vaporization<br />
sequence decontaminates the pass<br />
box, work station interior, centrifuge<br />
and CO2 incubator exterior and<br />
docking gaskets.<br />
• Once the vaporization is complete,<br />
the H2O2 program implements a<br />
dwell period to ensure that proper<br />
exposure times are maintained for<br />
8 CWPS <strong>Integrated</strong> <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong> www.sanyobiomedical.com<br />
Grade A<br />
In Operation 3,520<br />
Grade B<br />
In Operation 352,000<br />
At rest 3,520<br />
Grade C<br />
In Operation 3,520,000<br />
At rest 352,000<br />
Grade D<br />
At rest 3,520,000<br />
Grade A<br />
In Operation 3,500<br />
Grade B<br />
In Operation 350,000<br />
At rest 3,500<br />
Grade C<br />
In Operation 3,500,000<br />
At rest 350,000<br />
Grade D<br />
At rest 3,500,000<br />
Prepared<br />
<strong>Cell</strong>s<br />
a wide range of pathogens (contact<br />
SANYO for independent test results).<br />
• At the end of the pre-programmed<br />
dwell period a resolution process<br />
eliminates fumes and toxic residuals.<br />
Isolator Interchange<br />
• The interchange pass box allows safe<br />
access to the work area for supplies,<br />
instruments, devices, sterile media<br />
and labware.<br />
• When materials are brought into the<br />
work station they are first positioned<br />
inside the interchange for H2O2<br />
decontamination.<br />
• Decontamination is manually initiated<br />
and automatically sequenced once<br />
started.<br />
• When the H2O2<br />
vaporization process is<br />
complete, the inner door is opened and<br />
the transfer is completed.<br />
• Door interlocks permit simultaneous<br />
opening to protect the barrier isolator.
D<br />
00,000)<br />
SANYO <strong>CPWS</strong> Series Aseptic Environment<br />
Conventional GMP Cleanroom Facility (Open System)<br />
with Biological Safety Cabinets<br />
4 m2<br />
Class A<br />
(100)<br />
18 m2<br />
-152ºC<br />
10 m2<br />
18 m2<br />
-32ºC<br />
PS<br />
3 m2<br />
PS<br />
11 m2<br />
PS PS<br />
Class B<br />
(10,000)<br />
PS<br />
6 m2<br />
PS<br />
11 m2<br />
16 m2<br />
Class C<br />
IO(100,000)<br />
16 m2<br />
PS PS PS<br />
PS<br />
PS<br />
10 m2<br />
7 m2<br />
Class D<br />
AR(100,000)<br />
SANYO <strong>CPWS</strong>, Barrier Isolator<br />
with <strong>Integrated</strong> Systems<br />
The SANYO <strong>CPWS</strong> offers significant<br />
throughput potential in a comparatively<br />
small footprint.<br />
• The <strong>CPWS</strong> can be installed in manageable<br />
Grade D (Class 100,000)<br />
environment, providing biological and<br />
physical protection between the work<br />
and the user.<br />
PPLF-4205<br />
Aseptic level<br />
low (D)<br />
-152ºC<br />
Aseptic level<br />
mid (C)<br />
Aseptic level<br />
high (B)<br />
Aseptic level raised gradually<br />
Air lock<br />
General environment (not clean)<br />
Highest aspect level = Aseptic<br />
(safety cabinets, etc)<br />
Cleanroom:<br />
-32ºC<br />
The biological safety cabinets within the cleanroom<br />
are open systems which require a stricter background<br />
environment. Access in and out of the cleanroom<br />
must comply with gown-up protocols consistent with<br />
GMP facilities. Class 100 air is ultimately delivered<br />
to the work product in one or more biological safety<br />
cabinet(s) operating independent of the cleanroom.<br />
Decontamination is costly and time-consuming;<br />
changeover from one protocol to another is difficult<br />
and expensive.<br />
• Access through the glove ports<br />
improves user comfort.<br />
The integral H<br />
• 2O2 decontamination<br />
system is completed in less than two<br />
hours, permitting frequent changeover<br />
and assuring separation integrity from<br />
one cell process to the next.<br />
(A)<br />
9
SANYO <strong>CPWS</strong> Series Features<br />
CO2 Incubator<br />
The modular CO2 incubator is an adaptation<br />
of the full-performance SANYO<br />
MCO-5AC(IS). This incubator is designed<br />
for precise temperature and CO2 control<br />
with elevated relative humidity to minimize<br />
cell desiccation.<br />
• By using multiple, detachable CO2<br />
incubators, the <strong>CPWS</strong> can manage<br />
multiple patient protocols through<br />
complete product segregation,<br />
thereby assuring aseptic conditions<br />
and eliminating any possibility of<br />
cross-contamination or mishandling<br />
of patient-specific cells.<br />
• Segregation permits compliance<br />
with published FDA criteria associated<br />
with regenerative medicine and<br />
in vivo cell therapies.<br />
• The incubator attaches to a docking<br />
collar adjacent to the barrier isolator.<br />
• Once attached, the barrier isolator<br />
undergoes a 2-hour H2O2<br />
decontamination process before<br />
the incubator door is opened. This<br />
process sterilizes the work area,<br />
incubator face, centrifuge and<br />
pass-thru interchange.<br />
• The H2O2<br />
effectively decontaminates<br />
the CO2 connection.<br />
• When work is complete, the<br />
incubator is sealed, detached and<br />
moved to a user-defined staging<br />
location on a wheeled cart.<br />
• The next incubator can be moved into<br />
position to repeat the process for<br />
another patient.<br />
• There is no limit to the number of<br />
SANYO MCO-5AC(IS) incubators that<br />
can be used with the work station.<br />
10 CWPS <strong>Integrated</strong> <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong><br />
Incubator Features and Benefits<br />
The incubator is a modification of<br />
SANYO’s popular MCO-5AC. The compact<br />
1.4 cu.ft. (49 liter) interior chamber<br />
is constructed of SANYO’s exclusive<br />
inCu saFe copper-enriched stainless<br />
steel, creating a natural germicidal barrier<br />
against airborne contamination.<br />
• An integrated microprocessor controller<br />
supervises all functions including<br />
temperature and CO2 setpoints,<br />
control, alarm and monitoring.<br />
• The patented Direct Heat and<br />
Air Jacket control system assures<br />
temperature uniformity and stability<br />
essential to the most sensitive<br />
cell lines.<br />
• The rounded-corner, electropolished<br />
interior is configured for use with<br />
a variety of standard cell culture<br />
vessels.<br />
• All interior components are designed<br />
to withstand repeated H2O2 decontamination<br />
cycles expected in high<br />
turnover environments. Components<br />
are removable without tools.<br />
Modular Incubator<br />
Model MCO-5AC(IS)<br />
docked to the <strong>CPWS</strong><br />
Centrifuge<br />
The centrifuge is installed beneath<br />
the interior work surface and accessible<br />
under aseptic conditions without<br />
removing cells from the protected<br />
environment.<br />
• The position and orientation of<br />
the centrifuge assures thorough<br />
decontamination during the H2O2<br />
vaporization cycle.<br />
• The integrated design eliminates<br />
the requirement for additional floor<br />
space in a GMP environment.<br />
• A variety of fixed and swinging<br />
rotorsis available.<br />
• Centrifuge controls are located<br />
external to the work area at the<br />
front of the centrifuge module.<br />
<strong>CPWS</strong> Centrifuge
SANYO <strong>CPWS</strong> Series Features<br />
Decontamination Sequence<br />
Total Decontamination<br />
A total H2O2 vapor decontamination creates aseptic<br />
conditions in the interchange pass box, the glove box and<br />
(optional) centrifuge module.<br />
Pass Box Only<br />
Instruments or materials introduced into the glove box are<br />
decontaminated by a pass box cycle only, avoiding the need<br />
to decontaminate the entire glove box work area. <strong>Work</strong> can<br />
continue in the glove box while the cycle is in process.<br />
<strong>Cell</strong> Culture Incubator<br />
Incubator and <strong>Work</strong> Area. After aseptic protocols are<br />
completed, cultures are transferred to the (optional) CO2<br />
incubator where growth can continue under aseptic conditions<br />
after the incubator is detached. Unwanted materials<br />
are removed through the pass box interchange.<br />
Changeover<br />
When handling different cells from the previous protocol,<br />
the next (optional) cell culture incubator is docked to the<br />
work station glove box. The work station, interchange<br />
pass box (optional) centrifuge and incubator door are<br />
decontaminated prior to opening. The changeover process<br />
is completed in less than two hours.<br />
Decontaminated Contaminated<br />
Possible cell-derived<br />
contaminated<br />
One <strong>CPWS</strong> work station can service multiple CO2 incubators to permit easier<br />
cell segregation. One incubator is attached to the work station at the docking<br />
collar and subjected to a quick, safe H2O2 decontamination process. When<br />
work is completed, the incubator is sealed and returned to the staging area<br />
and another incubator is attached and the process is repeated.<br />
11
SANYO <strong>CPWS</strong> Series Features<br />
1<br />
<strong>Work</strong> station Glove Box<br />
The work station creates Grade A Class 100 air quality<br />
within the glove box, and permits installation within a<br />
Grade D Class 100,000 room, eliminating the need for<br />
a typical cleanroom. The self-contained, closed system<br />
protects both the user and the work product without the<br />
need for a second gowning. An ergonomically shaped<br />
front access is angled 6° for user comfort, glare<br />
reduction and reach throughout the work surface.<br />
<strong>Cell</strong> Monitoring System<br />
(Optional)<br />
An integrated cell monitoring system<br />
reduces additional footprint required in<br />
a GMP environment. <strong>Cell</strong> monitor components<br />
are located within the barrier isolator<br />
and designed to withstand repeated<br />
H2O2 decontamination sequences.<br />
• The interior components include<br />
a large, 19" LCD monitor, and a<br />
separate collection module.<br />
• The collection module includes<br />
a high-performance CCD camera<br />
(charge coupled device) with objective<br />
lens and phase difference filter.<br />
• The monitor displays real time images<br />
with total magnification of 110×.<br />
• Images can be captured via a noncontact<br />
photo sensor, 2.4 × 1.8 mm<br />
for monitor display and digital<br />
recording.<br />
• A standard personal computer<br />
external to the aseptic isolator work<br />
area is used for data acquisition and<br />
image management.<br />
1<br />
12 CWPS <strong>Integrated</strong> <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong> www.sanyobiomedical.com<br />
2<br />
Pass Box Decontamination<br />
3<br />
Decontamination System<br />
Materials are brought into the work chamber from the The <strong>CPWS</strong> includes an H2O2 decontamination system<br />
outside through the pass box interchange. The H2O2 that generates H2O2 vapor to produce validated results.<br />
decontamination is manually initiated and automatically This system covers the pass box interchange, glove<br />
sequenced, thoroughly sterilizing the pass thru material box work area, optional centrifuge and optional CO2<br />
and protecting the integrity of the work surface. The incubator. The enriched H2O2 solution is contained in<br />
interchange contamination process can function alone, a replaceable bottle and inserted as a cartridge inside<br />
independent of the glove box.<br />
the decontamination module.<br />
Operator’s<br />
Monitor<br />
Observation<br />
Module<br />
AC Power<br />
Control/Relay<br />
Box<br />
2<br />
Images from PC<br />
Camera IF<br />
Various Signals IF<br />
Lighting VCC, GND<br />
Light Dimming Signal<br />
Cameral IF (power, image signals)<br />
Power SW Signal<br />
Capture SW Signal<br />
3<br />
PC for Capturing
SANYO <strong>CPWS</strong> Model PPLF-4025 Specifications<br />
Component Specifications<br />
Component Description<br />
Barrier Isolator Base Unit<br />
Interchange Pass Box Included<br />
Airflow and HEPA Filtration System Included<br />
H2O2 System Included<br />
Centrifuge Optional<br />
CO2 Incubator Optional<br />
<strong>Cell</strong> Observation System Optional<br />
Centrifuge and Module<br />
System Summary Specifications<br />
Component Order Number<br />
Base <strong>Work</strong> <strong>Station</strong>,<br />
Barrier Isolator<br />
Feature Specification<br />
PPLF-4025<br />
Interchange Included<br />
HEPA Filtration System Included<br />
Centrifuge Optional<br />
<strong>Cell</strong> Observation Module Optional<br />
CO2 Incubator MCO-5AC(IS)<br />
Exterior Dimensions (w x f-b x h) 21.25" × 22.9" × 30.3" (540 x 582 x 771 mm)<br />
Maximum Speed 2100 RPM<br />
Maximum Centrifugal Acceleration 10 to 970G centrifugal acceleration settable range<br />
Controls, Alarms Externally mounted, front, with foot switch for cut-off.Door lock status displayed.<br />
Pre-Sets Up to 4 patterns in memory<br />
Time Settable Range 10 to 50 seconds (10 second increments); 1 to 99 minutes (1 minute increments); and infinite<br />
Acceleration/Deceleration Settings 3 stage<br />
Motor Shaft Stainless steel<br />
Capacity 1000ml (50ml tubes, × 20)<br />
13
SANYO <strong>CPWS</strong> Model PPLF-4025 Specifications<br />
Barrier Isolator<br />
Dimensions<br />
Overall Exterior Dimensions (w x f-b x h) 115" × 41" × 87.8" (2920 × 1045 × 2230 mm) net of attached optional incubator<br />
Interior Dimensions (w x f-b x h) 70.7" × 28.5" × 34" (1796 × 726 × 864 mm)<br />
Interior <strong>Work</strong> Surface Elevation 31.5" above floor (800 mm)<br />
Glove Elevation 41.3" above floor (1050 mm<br />
Pass Box Interchange (w x f-b x h) 19.6" × 16.7" × 15.8" (500 × 425 × 400 mm)<br />
Net Weight 2207 lbs/1000 kg, nominal<br />
Controls, Alarms<br />
Pressure Monitor Red LCD and audible alarm if pressure falls below alarm setpoint.<br />
Fan Motor Audible alarm during motor shutdown<br />
Construction<br />
Exterior Finish Painted steel<br />
Interior Finish Polished stainless steel<br />
Front Glove Port Panel, Angle 6° Polycarbonate with painted steel frame. Hinged for opening to load equipment.<br />
Air System<br />
Air Quality Grade A Class 100 over work surface; installation in a Grade D, Class 100,000 ambient room<br />
Airflow System and Velocity Vertical downflow over work surface, 0.2~0.3 m/s ± 20% @6" below supply filter screen (150 mm)<br />
Filtration System Independent HEPA supply and exhaust filters, efficiency 99.99% (0.3 µm PAO), scan tested and passed<br />
Filtration System, Pass Box Independent HEPA supply and exhaust filters, efficiency 99.99% (0.3 µm PAO), scan tested and passed<br />
Intake and Exhaust<br />
Electrically switched system. Room air intake thru HEPA supply filter;<br />
return exhaust to room thru HEPA exhaust filter<br />
Relative Air Pressure<br />
<strong>Work</strong> station<br />
Positive pressure after decontamination of work station and pass box;<br />
audible notification when ready, 60Pa<br />
Pass Box Interchange Positive pressure after decontamination of pass box only; audible notification when ready, 220Pa<br />
Interior <strong>Work</strong> Area<br />
Lighting Fluorescent lamps (3), 30w each, top of panel, top of right, left<br />
Electrical Outlet Duplex, drip-proof receptacle<br />
Pass Box Interchange<br />
Door Front opening to outside; left side opening to interior work area<br />
Material Polished stainless steel<br />
Decontamination System<br />
Function<br />
H2O2 Resolution and Aeration<br />
Decontamination Cycle Time<br />
14 CWPS <strong>Integrated</strong> <strong>Cell</strong> <strong>Processing</strong> <strong>Work</strong> <strong>Station</strong> www.sanyobiomedical.com<br />
H2O2 vaporization; simultaneous decontamination of interior barrier isolator work station,<br />
pass box interchange, optional centrifuge and optional CO2 incubator<br />
Catalytic, external to the work area. Residual H2O2 density at catalytic outlet is at or below 1ppm<br />
(by TLV-TWA). Fresh, HEPA filtered air follows resolution<br />
Load dependent; typical cycle times range from 1.0 to 2.0 hours<br />
from manual initiation of automatic sequence.<br />
Utilities<br />
Electrical Main system, 4500w, 220V, AC, 60Hz. Electrical outlet 120V, AC, 60Hz.
SANYO <strong>CPWS</strong> Model PPLF-4025 Specifications<br />
CO 2 Incubator (Modular), SANYO MCO-5AC(IS)<br />
Dimensions<br />
Exterior Dimensions Without Stand (w x f-b x h) 23.5"W× 22.1" × 24.7" (597 × 561 × 626 mm)<br />
Interior Dimensions (w x f-b x h) 13.8" × 14.9" × 14.8" (350 × 378 × 375 mm)<br />
Net Weight, Nominal 110 lbs/50 kg<br />
Volume 1.7 cu.ft./49 liters nominal<br />
Operating System<br />
CO2 Sensor Thermal<br />
InCu SaFe Copper Enriched Stainless Steel Interior Standard<br />
Microprocessor Controller/Display, Door Mounted Standard<br />
Direct Heat, Air (DHA) Air Jacket Standard<br />
Sterilization and Decontamination<br />
H2O2 Decontamination When docked to work station<br />
Standard, Copper Enriched Stainless Steel Interior Germicidal protection<br />
Environmental Performance<br />
Temperature Control Range +7.0°C above ambient to 50°C in a 5°C to 35°C ambient<br />
Temperature Control Uniformity and Deviation ±0.25°C in 25°C ambient, setting 37°C, 5% CO2, no load<br />
CO2 Control Range and Deviation 0% to 20%, ±0.15% in 25°C ambient, setting 37°C, 5% CO2, no load<br />
Airflow Continuous with inner door closed<br />
Interior Humidity<br />
95%RH @ 37°C through evaporation via DHA heating system;<br />
optional reflective/deflective optical low water sensor<br />
Control, Monitoring, Alarm<br />
Temperature and CO2 Control Setpoint resolution 0.1°, 0.1%<br />
Display LED, resolution 0.1°, 0.1%<br />
Communications Optional 4-20mA connection; Optional PC interface MTR-480<br />
Cabinet Design and Construction<br />
Superstructure, Exterior Cabinet and Door Galvanized steel exterior, baked-on enamel finish<br />
Interior and Shelves Copper-enriched stainless steel<br />
Inner Door Tempered glass<br />
Insulation Rigid foam polyurethane<br />
Outer Door Side oriented for docking with work station<br />
Access Port Single, 1.18"/30mm with silicone stopper<br />
Leveling Feet Adjustable<br />
Energy, Electrical, Utilities<br />
Maximum Power Consumption 205W<br />
Maximum Heat Discharge 740kJ/h<br />
Electrical 115V,60Hz<br />
CO2 Gas Connection 4 to 6 mm barbed fitting<br />
CO2 Gas Input Pressure Nominal 0.03 MPaG/0.3kg/cm2/5.0lbs/sq.in; from two-stage CO2 regulator<br />
15
SANYO Service and Support<br />
Unique SANYO Services<br />
• On-site consultation<br />
Model PPLF-4205<br />
• Specialized documentation for each<br />
individual unit<br />
• Customized testing procedures<br />
based on personalized customer<br />
requirements<br />
SANYO Connect<br />
SANYO’s customer-driven biomedical<br />
service program guarantees local<br />
attention from qualified SANYO service<br />
representatives, whenever and wherever<br />
you need it.<br />
• New Unit Installation and Training<br />
• Preventative Maintenance<br />
• Warranty and Non-Warranty Repairs<br />
• Calibration/Validation Services<br />
• Refurbishment and Reconditioning<br />
• Customized Service and Warranty<br />
Programs<br />
• In-Stock Parts for Immediate Delivery<br />
Safe<strong>Cell</strong> UV U.S. Patent 6255103; Direct Heat and Air Jacket U.S. Patent 5519188;<br />
Safe<strong>Cell</strong> UV, inCu saFe , Direct Heat and Air Jacket , P.I.D./R and Active Background<br />
Contamination Control , are trademarks of SANYO Electric Biomedical Co., Ltd.<br />
© 2010 Specifications subject to change without notice.<br />
Pre-Delivery and On-Site Services<br />
Pre-delivery services include factory<br />
acceptance testing, calibration, and<br />
temperature mapping. On-site services<br />
include installation qualification,<br />
operational qualification, performance<br />
qualification, calibration and temperature<br />
mapping.<br />
SANYO North America Corporation<br />
Biomedical Solutions Division<br />
1300 Michael Drive, Suite A, Wood Dale, IL 60191 USA<br />
Toll-Free 800-858-8442 Fax 630-238-0074<br />
www.sanyobiomedical.com