Nucleus® 5 Cochlear Implant (CI512) - For professionals - Cochlear ...
Nucleus® 5 Cochlear Implant (CI512) - For professionals - Cochlear ...
Nucleus® 5 Cochlear Implant (CI512) - For professionals - Cochlear ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
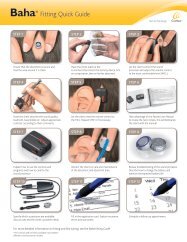
RECEIVER STIMULATOR<br />
General Features<br />
• Weight - 8.8 g (incl. electrode array).<br />
• Designed to be impact resistant and 2.5x stronger *<br />
MRI<br />
• MRI safe at 1.5 Tesla with magnet removed<br />
(for further details refer to the Surgeon’s guide).<br />
ELECTRODE ARRAY<br />
Contacts<br />
• 22 half-banded platinum electrodes - molded in a<br />
perimodiolar shape.<br />
• Electrode contacts arranged in non-uniform spacing from<br />
0.4 to 0.8 mm and spaced over 15 mm active array.<br />
• More robust lead designed to withstand the rigors of<br />
implantation for a lifetime.<br />
General Features<br />
• Platinum, arrow stylet - holds the electrode straight<br />
for insertion with Advance Off-Stylet (AOS )<br />
surgical technique.<br />
• AOS surgical technique and Softip electrode<br />
- minimize lateral wall insertion force.<br />
• Two extracochlear electrodes - one titanium plate<br />
at the implant receiver stimulator and a separate<br />
0.6 mm diameter cylindrical electrode.<br />
• A white marker between 10th and 11th array contact<br />
- indicates insertion depth when the tip is close to the<br />
lateral wall of the cochlea.<br />
Dimensions<br />
ARROW STYLET<br />
• 19 mm intracochlear length including Softip.<br />
• Electrode diameter at apical end - 0.5 mm.<br />
• Electrode diameter at basal end - 0.8 mm.<br />
1 Nucleus Reliability Report, June 2008<br />
2 Advanced Bionics - Auditory Reliability Report, Summer 2008. 2.5 years of data,<br />
launched in 2005.<br />
3 MED-EL Website: http://www.medel.com/english/img/animations/SONATA_CSR.swf<br />
April 2009 21 months of data, launched in 2007.<br />
4 Parkinson AJ, Arcaroli J, Staller SJ , Arndt PL, Cosgriff A, Ebinger K. The nucleus 24 contour<br />
cochlear implant system: adult clinical trial results. Ear Hear. 2002 Feb;23(1 Suppl):41S-48S.<br />
5 US patent 7, 184, 843. [On file] 2007 Feb 27.<br />
COCHLEOSTOMY<br />
LOCATION (RANGE)<br />
13 mm<br />
<strong>Cochlear</strong>, the elliptical logo, AutoNRT, Advance-Off-Stylet, AOS, Freedom and Softip are<br />
trademarks of <strong>Cochlear</strong> Limited. Nucleus is a registered trademark of <strong>Cochlear</strong> Limited.<br />
*The Nucleus 5 <strong>Cochlear</strong> <strong>Implant</strong> (<strong>CI512</strong>) passed impact testing at 2.5 times the strength<br />
the Freedom implant was tested to.<br />
19 mm<br />
Softip<br />
MICROELECTRONIC PLATFORM<br />
General Features<br />
• Power efficient, custom design.<br />
• Amplitude range: 10 uA to 1.75 mA.<br />
• Stimulation rates up to 31.5 kHz.<br />
• Pulse width: 12 us to 400 us per phase.<br />
• <strong>Implant</strong> ID to uniquely identify implants and to avoid<br />
unintended stimulation.<br />
Stimulation Modes<br />
• Monopolar, bipolar mode and common ground<br />
stimulation, biphasic current pulses.<br />
Telemetry Capability<br />
• Ultra-low-noise floor (~1 μV) - enabling advanced<br />
AutoNRT telemetry capabilities.<br />
• Includes fully integrated Electrophysiology telemetry<br />
modes - NRT, AutoNRT, ESRT, ABR, CEP and<br />
intraoperative NRT.<br />
www.cochlear.com<br />
<strong>Cochlear</strong> Americas<br />
13059 East Peakview Avenue<br />
Centennial, CO 80111 USA<br />
Not all patients with hearing loss are candidates for cochlear implantation. <strong>Cochlear</strong><br />
implantation is a surgical procedure, and carries with it the risks typical for surgery. <strong>For</strong><br />
complete information regarding indications, warnings and adverse effects,<br />
please refer to the Nucleus Freedom Package Insert (available at<br />
www.cochlearamericas.com/NucleusIndications).<br />
© <strong>Cochlear</strong> Limited 2009<br />
FUN1007 ISS3 AUG09<br />
50.5 mm<br />
30.5 mm<br />
23.5 mm<br />
22.3 mm<br />
All measurements include silicone over-molding<br />
Tel: 1 303 790 9010<br />
Fax: 1 303 792 9025<br />
Toll Free: 1 800 523 5798<br />
FUN1007 ISS3 AUG09 Tech Specs.indd 2 8/27/09 5:47:37 PM