Strategy For Limiting Risks Human Health Draft of ... - ECHA - Europa
Strategy For Limiting Risks Human Health Draft of ... - ECHA - Europa
Strategy For Limiting Risks Human Health Draft of ... - ECHA - Europa
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
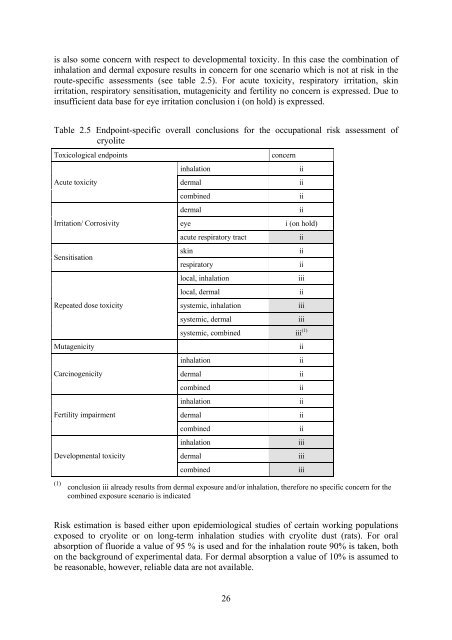
is also some concern with respect to developmental toxicity. In this case the combination <strong>of</strong><br />
inhalation and dermal exposure results in concern for one scenario which is not at risk in the<br />
route-specific assessments (see table 2.5). <strong>For</strong> acute toxicity, respiratory irritation, skin<br />
irritation, respiratory sensitisation, mutagenicity and fertility no concern is expressed. Due to<br />
insufficient data base for eye irritation conclusion i (on hold) is expressed.<br />
Table 2.5 Endpoint-specific overall conclusions for the occupational risk assessment <strong>of</strong><br />
cryolite<br />
Toxicological endpoints concern<br />
Acute toxicity<br />
Irritation/ Corrosivity<br />
Sensitisation<br />
Repeated dose toxicity<br />
inhalation ii<br />
dermal ii<br />
combined ii<br />
dermal ii<br />
eye i (on hold)<br />
acute respiratory tract ii<br />
skin ii<br />
respiratory ii<br />
local, inhalation iii<br />
local, dermal ii<br />
systemic, inhalation iii<br />
systemic, dermal iii<br />
systemic, combined iii (1)<br />
Mutagenicity ii<br />
Carcinogenicity<br />
Fertility impairment<br />
Developmental toxicity<br />
inhalation ii<br />
dermal ii<br />
combined ii<br />
inhalation ii<br />
dermal ii<br />
combined ii<br />
inhalation iii<br />
dermal iii<br />
combined iii<br />
(1) conclusion iii already results from dermal exposure and/or inhalation, therefore no specific concern for the<br />
combined exposure scenario is indicated<br />
Risk estimation is based either upon epidemiological studies <strong>of</strong> certain working populations<br />
exposed to cryolite or on long-term inhalation studies with cryolite dust (rats). <strong>For</strong> oral<br />
absorption <strong>of</strong> fluoride a value <strong>of</strong> 95 % is used and for the inhalation route 90% is taken, both<br />
on the background <strong>of</strong> experimental data. <strong>For</strong> dermal absorption a value <strong>of</strong> 10% is assumed to<br />
be reasonable, however, reliable data are not available.<br />
26