ReOss® Powder - Intra-Lock
ReOss® Powder - Intra-Lock ReOss® Powder - Intra-Lock
ReOss ® Powder Indication ReOss ® is indicated for use in filling and/or augmenting intraoral/maxillofacial osseous defects; such as infrabony/intrabony periodontal osseous defects, furcation defects, alveolar ridge osseous defects, tooth extraction sites and in sinus elevation procedures. Sub-Micron HA Particles can begin to be seen permeating the PGLA Matrix at 50,000x. Red highlights are used here as visual aid. SEM Image of ReOss ® Biocomposite 300x Showing Internal Structure of an Individual Granule ReOss® Sub-Micron HA Infused Synthetic Biomaterial ReOss® has a multi-pore three-dimensional architecture that creates an environment for new bone growth. The scaffold also provides a hospitable adhesive substrate that serves as a strong physical support for the infusion and growth of bone cells. The entire structure is an intricate, highly interconnected matrix with enhanced hydrophilic properties. Through a HA HA HA HA SEM Image of ReOss ® Biocomposite 10,000x Showing Porous Nature of the PLGA Matrix patented process utilizing barosynthesis, the biomaterial’s highly porous, synthetic polymer foam is permeated with osteoconductive sub-micron sized particles of HA. ReOss’s bone-like foam scaffold, osteoconductivity, and increased hydrophilic surface provide an environment for the stimulation of bone regeneration. Technical Properties ReOss ReOss® Bone Bone Growth Growth Initiator Initiator is a is high a high purity purity biocomposite biocomposite which which is synthesized is synthesized utilizing utilizing a special a special process process involving involving high high pressure pressure formation. formation. Typical Composition Purity Porosity Pore Size Particle Size Size Resorption Time Time 50% Hydroxyapatite (Ca ( Ca10(PO4)6 10(PO4) 6(OH) (OH)2) 2) 50% Poly(lactide-co-glycolide) < 70 % Macropores = 15-300 150- 300 microns Micropores < ≤10 10 microns 300-750 500 -1000 Microns microns From 6 to 12 Months Available Configurations Catalogue No. No. Volume Reference List ReOss® ReOssPowder: Powder: ReOss® ReOssPutty: Putty: ReOss ReOss® Injectable Gel: 1. 1. Poly Poly (lactide-co-glycolide)/hydroxyapatite (lactide-co-glycolide)/hydroxyapatite composite composite scaffolds for bone tissue tissue engineering. engineering. Kim Kimet et al. al. Biomaterials Biomaterials 27(2006) 27(2006) 1399-1409. 1399-1409. 2. A poly (lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. 2. A poly (lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. Kim et al. Journal of Biomedical Research Part A Vol 80A Issue 1, pp 206-215. Kim et al. Journal of Biomedical Research Part A Vol 80A Issue 1, pp 206-215. 3. Comparison of Osteogenic Potential Between Apatite-Coated Poly (lactide-co-glycolide)/Hydroxy apatite 3. Comparison Particulates of Osteogenic and Bio-Oss. Potential Kim et Between al. Dental Apatite-Coated Materials Journal Poly 2008; (lactide-co-glycolide)/Hydroxy- 27(3): 368-375. 4. apatite Peripheral Particulates nerve regeneration and Bio-Oss. within Kim et al. an Dental asymmetrically Materials porous Journal PLGA/ 2008; Pluronic 27(3): 368-375. F127 nerve guide conduit. 4. Peripheral Oh et nerve al. Biomaterials regeneration 29(2008) within 1601-1609 an asymmetrically porous PLGA/ Pluronic F127 nerve guide 5. conduit. Sterilization, Oh ettoxicity, al. Biomaterials biocompatibility 29(2008) 1601-1609 and clinical applications of polylactic acid/polyglycolic acid copolymers. 5. Sterilization, Athanasiou toxicity, biocompatibility et al. Biomaterials and 17 clinical (1996) applications 93-102 of polylactic acid/polyglycolic acid 6. copolymers. In vitro biocompatibility Athanasiou et of al. bioresorbable Biomaterials 17 polymers: (1996) 93-102 poly(L-lactide) and poly(lactide-co-glycolide). A. Ignatius, L. E. Claes Biomaterials, Volume 17, Issue 8, 1996, Pages 831-839 6. In vitro biocompatibility of bioresorbable polymers: poly(L-lactide) and poly(lactide-co-glycolide). A. Ignatius, L. E. Claes Biomaterials, Volume 17, Issue 8, 1996, Pages 831-839 RP-1 0.5cc 0.5 cc RP-3 RP-3 1cc 1cc RP-2 0.5cc 0.5 cc RP-4 RP-4 1cc1 cc RG-1 0.5cc 0.5 cc RG-2 RG-2 1cc1 cc Color coded container caps for easier product recognition •••••• SUB-MICRON HA INFUSED SYNTHETIC BIOMATERIAL 7. 7. Biodegradation Biodegradation and and biocompatibility biocompatibility of of PLA PLA and and PLGA PLGA microspheres. James M. Anderson, Matthew Matthew S. S. Shiva Shiva Advanced Advanced Drug Drug Delivery Delivery Reviews Reviews Volume Volume 28, 28, Issue Issue 1, 1, 13 13 October October 1997, 1997, Pages Pages 5-24 5-24 8. Resorbability of bone substitute biomaterials by human osteoclasts. Schilling et al. Biomaterials, 8. Resorbability of bone substitute biomaterials by human osteoclasts. Schilling et al. Biomaterials, Volume 25, Issue 18, Aug 2004, pp 3963-3972. Volume 25, Issue 18, Aug 2004, pp 3963-3972. 9. The biodegradation of hydroxyapatite bone graft substitutes in vivo. Rumpel et al. Folia Morphology 9. Volume The biodegradation 65, No 1, pp 43-48 of hydroxyapatite bone graft substitutes in vivo. Rumpel et al. Folia Morphology Volume 10. Biocompatibility 65, No 1, pp 43-48 of Scaffold Components and Human Bone Fetal Cells. Montjovent et al. European 10. Cells Biocompatibility and Material Vol.5. of Scaffold Suppl. Components 2,2003 p.79 and Human Bone Fetal Cells. Montjovent et al. European Cells 11. Biodegradable and Material Vol.5. and Suppl. bioactive 2,2003 porous p.79polymer/inorganic composite scaffolds for bone tissue 11. engineering. Biodegradable Rezwan and et bioactive al. Biomaterials porous polymer/inorganic 27(2006) 3413-3431 composite scaffolds for bone tissue engineering. 12. Physico/Chemical Rezwan et characterization, al. Biomaterials 27(2006) in vitro and 3413-3431 in vivo evaluation of ReOss® and Synthograft particulate grafting materials. Coimbra et al. 12. Physico/Chemical characterization, in vitro and in vivo evaluation of ReOss and Synthograft particulate grafting materials. Coimbra et al.
ReOss ® <strong>Powder</strong><br />
Indication<br />
ReOss ® is indicated for use in filling and/or augmenting intraoral/maxillofacial osseous defects; such as infrabony/intrabony<br />
periodontal osseous defects, furcation defects, alveolar ridge osseous defects, tooth extraction sites and in sinus elevation<br />
procedures.<br />
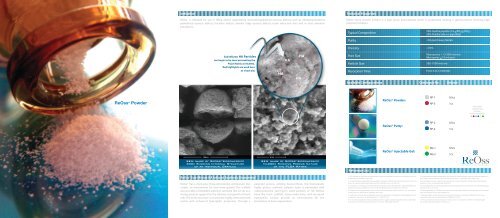
Sub-Micron HA Particles<br />
can begin to be seen permeating the<br />
PGLA Matrix at 50,000x.<br />
Red highlights are used here<br />
as visual aid.<br />
SEM Image of ReOss ® Biocomposite<br />
300x Showing Internal Structure<br />
of an Individual Granule<br />
<strong>ReOss®</strong> Sub-Micron HA Infused Synthetic Biomaterial<br />
<strong>ReOss®</strong> has a multi-pore three-dimensional architecture that<br />
creates an environment for new bone growth. The scaffold<br />
also provides a hospitable adhesive substrate that serves as a<br />
strong physical support for the infusion and growth of bone<br />
cells. The entire structure is an intricate, highly interconnected<br />
matrix with enhanced hydrophilic properties. Through a<br />
HA<br />
HA<br />
HA<br />
HA<br />
SEM Image of ReOss ® Biocomposite<br />
10,000x Showing Porous Nature<br />
of the PLGA Matrix<br />
patented process utilizing barosynthesis, the biomaterial’s<br />
highly porous, synthetic polymer foam is permeated with<br />
osteoconductive sub-micron sized particles of HA. ReOss’s<br />
bone-like foam scaffold, osteoconductivity, and increased<br />
hydrophilic surface provide an environment for the<br />
stimulation of bone regeneration.<br />
Technical Properties<br />
ReOss<br />
<strong>ReOss®</strong><br />
Bone<br />
Bone<br />
Growth<br />
Growth<br />
Initiator<br />
Initiator<br />
is a<br />
is<br />
high<br />
a high<br />
purity<br />
purity<br />
biocomposite<br />
biocomposite<br />
which<br />
which<br />
is synthesized<br />
is synthesized<br />
utilizing<br />
utilizing<br />
a special<br />
a special<br />
process<br />
process<br />
involving<br />
involving<br />
high<br />
high<br />
pressure pressure formation. formation.<br />
Typical Composition<br />
Purity<br />
Porosity<br />
Pore Size<br />
Particle Size Size<br />
Resorption Time Time<br />
50% Hydroxyapatite (Ca ( Ca10(PO4)6 10(PO4) 6(OH) (OH)2) 2)<br />
50% Poly(lactide-co-glycolide)<br />
< 70 %<br />
Macropores = 15-300 150- 300 microns<br />
Micropores < ≤10 10 microns<br />
300-750 500 -1000 Microns microns<br />
From 6 to 12 Months<br />
Available Configurations Catalogue No. No. Volume<br />
Reference List<br />
<strong>ReOss®</strong> ReOss<strong>Powder</strong>: <strong>Powder</strong>:<br />
<strong>ReOss®</strong> ReOssPutty: Putty:<br />
ReOss <strong>ReOss®</strong> Injectable Gel:<br />
1. 1. Poly Poly (lactide-co-glycolide)/hydroxyapatite (lactide-co-glycolide)/hydroxyapatite composite composite scaffolds for bone tissue tissue engineering. engineering. Kim Kimet et<br />
al.<br />
al.<br />
Biomaterials<br />
Biomaterials<br />
27(2006)<br />
27(2006)<br />
1399-1409.<br />
1399-1409.<br />
2. A poly (lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity.<br />
2. A poly (lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity.<br />
Kim et al. Journal of Biomedical Research Part A Vol 80A Issue 1, pp 206-215.<br />
Kim et al. Journal of Biomedical Research Part A Vol 80A Issue 1, pp 206-215.<br />
3. Comparison of Osteogenic Potential Between Apatite-Coated Poly (lactide-co-glycolide)/Hydroxy<br />
apatite 3. Comparison Particulates of Osteogenic and Bio-Oss. Potential Kim et Between al. Dental Apatite-Coated Materials Journal Poly 2008; (lactide-co-glycolide)/Hydroxy-<br />
27(3): 368-375.<br />
4.<br />
apatite<br />
Peripheral<br />
Particulates<br />
nerve regeneration<br />
and Bio-Oss.<br />
within<br />
Kim et al.<br />
an<br />
Dental<br />
asymmetrically<br />
Materials<br />
porous<br />
Journal<br />
PLGA/<br />
2008;<br />
Pluronic<br />
27(3): 368-375.<br />
F127 nerve guide<br />
conduit. 4. Peripheral Oh et nerve al. Biomaterials regeneration 29(2008) within 1601-1609<br />
an asymmetrically porous PLGA/ Pluronic F127 nerve guide<br />
5. conduit. Sterilization, Oh ettoxicity, al. Biomaterials biocompatibility 29(2008) 1601-1609 and clinical applications of polylactic acid/polyglycolic acid<br />
copolymers.<br />
5. Sterilization,<br />
Athanasiou<br />
toxicity, biocompatibility<br />
et al. Biomaterials<br />
and<br />
17<br />
clinical<br />
(1996)<br />
applications<br />
93-102<br />
of polylactic acid/polyglycolic acid<br />
6. copolymers. In vitro biocompatibility Athanasiou et of al. bioresorbable Biomaterials 17 polymers: (1996) 93-102 poly(L-lactide) and poly(lactide-co-glycolide).<br />
A. Ignatius, L. E. Claes Biomaterials, Volume 17, Issue 8, 1996, Pages 831-839<br />
6. In vitro biocompatibility of bioresorbable polymers: poly(L-lactide) and poly(lactide-co-glycolide).<br />
A. Ignatius, L. E. Claes Biomaterials, Volume 17, Issue 8, 1996, Pages 831-839<br />
RP-1 0.5cc 0.5 cc<br />
RP-3 RP-3<br />
1cc 1cc<br />
RP-2 0.5cc 0.5 cc<br />
RP-4 RP-4<br />
1cc1<br />
cc<br />
RG-1 0.5cc 0.5 cc<br />
RG-2 RG-2<br />
1cc1<br />
cc<br />
Color coded<br />
container caps<br />
for easier<br />
product recognition<br />
••••••<br />
SUB-MICRON HA INFUSED SYNTHETIC BIOMATERIAL<br />
7. 7. Biodegradation Biodegradation and and biocompatibility biocompatibility of of PLA PLA and and PLGA PLGA microspheres. James M. Anderson, Matthew Matthew S. S.<br />
Shiva<br />
Shiva<br />
Advanced<br />
Advanced<br />
Drug<br />
Drug<br />
Delivery<br />
Delivery<br />
Reviews<br />
Reviews<br />
Volume<br />
Volume<br />
28,<br />
28,<br />
Issue<br />
Issue<br />
1,<br />
1,<br />
13<br />
13<br />
October<br />
October<br />
1997,<br />
1997,<br />
Pages<br />
Pages<br />
5-24<br />
5-24<br />
8. Resorbability of bone substitute biomaterials by human osteoclasts. Schilling et al. Biomaterials,<br />
8. Resorbability of bone substitute biomaterials by human osteoclasts. Schilling et al. Biomaterials,<br />
Volume 25, Issue 18, Aug 2004, pp 3963-3972.<br />
Volume 25, Issue 18, Aug 2004, pp 3963-3972.<br />
9. The biodegradation of hydroxyapatite bone graft substitutes in vivo. Rumpel et al. Folia Morphology<br />
9. Volume The biodegradation 65, No 1, pp 43-48 of hydroxyapatite bone graft substitutes in vivo. Rumpel et al. Folia Morphology<br />
Volume<br />
10. Biocompatibility<br />
65, No 1, pp 43-48<br />
of Scaffold Components and Human Bone Fetal Cells. Montjovent et al. European<br />
10. Cells Biocompatibility and Material Vol.5. of Scaffold Suppl. Components 2,2003 p.79 and Human Bone Fetal Cells. Montjovent et al. European<br />
Cells 11. Biodegradable and Material Vol.5. and Suppl. bioactive 2,2003 porous p.79polymer/inorganic<br />
composite scaffolds for bone tissue<br />
11.<br />
engineering.<br />
Biodegradable<br />
Rezwan<br />
and<br />
et<br />
bioactive<br />
al. Biomaterials<br />
porous polymer/inorganic<br />
27(2006) 3413-3431<br />
composite scaffolds for bone tissue<br />
engineering. 12. Physico/Chemical Rezwan et characterization, al. Biomaterials 27(2006) in vitro and 3413-3431 in vivo evaluation of <strong>ReOss®</strong> and Synthograft<br />
particulate grafting materials. Coimbra et al.<br />
12. Physico/Chemical characterization, in vitro and in vivo evaluation of ReOss and Synthograft<br />
particulate grafting materials. Coimbra et al.
Barosynthesized BioComposite<br />
• 100% Synthetic<br />
• Osteo Adhesive Topography<br />
• Strong 3-D Scaffold<br />
• Osteoconductive<br />
• Enhanced Hydrophilicity<br />
<strong>ReOss®</strong> is hydrophilic and configured as a multi-pore three-dimensional scaffold engineered to integrate with the<br />
physiochemical state of bone tissue.<br />
Overview of <strong>ReOss®</strong>: A Resorbable Bone-like Biocomposite PLGA/HA:<br />
Poly (lactic-co-glycolic) acid / Hydroxyapatite<br />
<strong>ReOss®</strong> is a composite biomaterial comprised of two phases - a PLGA biodegradable polymer and a bioceramic. The polymer<br />
provides a structurally stable, porous and biocompatible 5,6,7 three-dimensional matrix to which biological fluids can penetrate,<br />
and cells can adhere.The HA bioceramic, due to its chemical and structural similarity to the mineral phase of native bone, allows<br />
the biocomposite to create a bond with the living host bone. 2,11<br />
Sub-Micron-HA Particles<br />
In order to improve the bioactivity of the ceramic phase, <strong>ReOss®</strong> utilizes Sub-Micron-sized particulate Hydroxyapatite (HA).<br />
This particulate size HA shows improved osteointegration and faster degradation times over larger particulate HA, which<br />
can impede bone growth because of its slow biodegradation. 1,2 Sub-Micron-sized HA also has been reported to augment<br />
protein adsorption and cell adhesion, further improving the ability for bone to regenerate. 2<br />
Multi-pore Resorbable Structure<br />
The porosity of the polymer matrix of <strong>ReOss®</strong> also provides an excellent environment to aid in stimulating bone regeneration.<br />
Through a patented process involving high-pressure formation of the polymer matrix, <strong>ReOss®</strong> is replete with both macro and<br />
micropores. The micropores allow biological fluids and small molecules, which aid in cell growth to perfuse the matrix,<br />
enveloping and sustaining the osteogenic cells that attach to the macropores of the scaffolds. As the cells begin to grow and<br />
develop, both phases of the biocomposite degrade, leaving behind a stable, natural bone matrix. 7,8,9<br />
Osteodynamics<br />
Several studies have shown that biodegradable polymer/bioceramic composites can improve bone regeneration as<br />
compared with conventional composites by optimizing controlled resorption, osteogenesis and osteointegration. 1,2,11 By<br />
controlling the parameters which determine the characteristics of resorption and osteoinductivity, <strong>ReOss®</strong> provides a superior<br />
vector for the stimulation of new bone growth.<br />
GLOBAL HEADQUARTERS<br />
6560 West Rogers Circle, Bldg. 24<br />
Boca Raton, Florida 33487 USA<br />
GLOBAL Tel: 877-330-0338<br />
HEADQUARTERS<br />
www.intra-lock.com • info@intra-lock.com<br />
6560 West Rogers Circle, Bldg. 24 • Boca Raton, Florida 33487 USA<br />
Tel: 877-330-0338<br />
www.intra-lock.com • info@intra-lock.com<br />
<strong>Intra</strong>-<strong>Lock</strong><br />
<strong>Intra</strong>-<strong>Lock</strong>® is registered trademark of <strong>Intra</strong>-<strong>Lock</strong>® International, Inc.<br />
U.S. & Foreign Patents Pending.<br />
® and ReOss ® are registered trademarks of <strong>Intra</strong>-<strong>Lock</strong> ® International, Inc.<br />
U.S. & Foreign Patents Pending.<br />
GLOBAL HEADQUARTERS<br />
6560 West Rogers Circle, Bldg. 24 • Boca Raton, Florida 33487 USA<br />
Tel: 877-330-0338<br />
www.intra-lock.com • info@intra-lock.com<br />
<strong>Intra</strong>-<strong>Lock</strong>® is registered trademark of <strong>Intra</strong>-<strong>Lock</strong>® International, Inc.<br />
U.S. & Foreign Patents Pending.<br />
CE 0499 0499 • EC • Rep: EC Rep: <strong>Intra</strong>-<strong>Lock</strong> System Europa, Spa., Srl., I-84100 Salerno • • © © Copyright 2008, 2009, <strong>Intra</strong>-<strong>Lock</strong>® <strong>Intra</strong>-<strong>Lock</strong> System International • S4EN-08-10CE<br />
0499 • EC Rep: <strong>Intra</strong>-<strong>Lock</strong> System Europa, Spa., I-84100 Salerno • © Copyright 2008, <strong>Intra</strong>-<strong>Lock</strong>® System International • S4EN-08-10<br />
® CE<br />
System International • S4EN-09-07<br />
SUB-MICRON HA INFUSED SYNTHETIC BIOMATERIAL