A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
62<br />
Effectiveness<br />
significant advantages over sequential<br />
mono<strong>the</strong>rapy with DMARDs or step-up<br />
combination DMARD use.<br />
Meta-analysis <strong>of</strong> infliximab results<br />
The principles <strong>of</strong> analysis and data presentation <strong>of</strong><br />
infliximab trials are <strong>the</strong> same as described in <strong>the</strong><br />
section ‘Data analysis’ (p. 14), towards <strong>the</strong><br />
beginning <strong>of</strong> this chapter.<br />
Infliximab versus o<strong>the</strong>r active treatment<br />
The licence for infliximab stipulates that<br />
infliximab has to be used in conjunction with<br />
methotrexate, thus head-to-head comparison<br />
between infliximab and methotrexate is not<br />
considered here. However, relevant data from a<br />
small, dose-ranging study 137 are summarised in<br />
Table 74 (Appendix 4). Infliximab 3 mg kg –1 at 0,<br />
2 and 6 weeks was more effective in all efficacy<br />
outcomes than a single infusion <strong>of</strong><br />
methylprednisolone (1 g i.v.) in a small open-label<br />
RCT by Durez and colleagues. 139<br />
Infliximab versus placebo (with concurrent,<br />
ongoing methotrexate)<br />
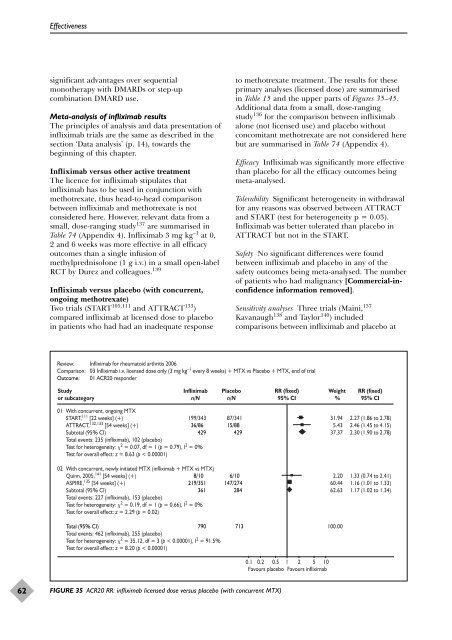
Two trials (START 105,111 and ATTRACT 133 )<br />
compared infliximab at licensed dose to placebo<br />
in patients who had had an inadequate response<br />
to methotrexate treatment. The results for <strong>the</strong>se<br />
primary analyses (licensed dose) are summarised<br />
in Table 15 and <strong>the</strong> upper parts <strong>of</strong> Figures 35–45.<br />
Additional data from a small, dose-ranging<br />
study 136 for <strong>the</strong> comparison between infliximab<br />
alone (not licensed use) and placebo without<br />
concomitant methotrexate are not considered here<br />
but are summarised in Table 74 (Appendix 4).<br />
Efficacy Infliximab was significantly more effective<br />
than placebo for all <strong>the</strong> efficacy outcomes being<br />
meta-analysed.<br />
Tolerability Significant heterogeneity in withdrawal<br />
for any reasons was observed between ATTRACT<br />
and START (test for heterogeneity p = 0.03).<br />
Infliximab was better tolerated than placebo in<br />
ATTRACT but not in <strong>the</strong> START.<br />
Safety No significant differences were found<br />
between infliximab and placebo in any <strong>of</strong> <strong>the</strong><br />
safety outcomes being meta-analysed. The number<br />
<strong>of</strong> patients who had malignancy [Commercial-inconfidence<br />
information removed].<br />
Sensitivity analyses Three trials (Maini, 137<br />
Kavanaugh 138 and Taylor 140 ) included<br />
comparisons between infliximab and placebo at<br />
Review: Infliximab for rheumatoid arthritis 2006<br />
Comparison: 03 Infliximab i.v. licensed dose only (3 mg kg –1 every 8 weeks) + MTX vs Placebo + MTX, end <strong>of</strong> trial<br />
Outcome: 01 ACR20 responder<br />
Study<br />
or subcategory<br />
Infliximab<br />
n/N<br />
Placebo<br />
n/N<br />
01 With concurrent, ongoing MTX<br />
START, 111 [22 weeks] (+)<br />
ATTRACT, 132,133 [54 weeks] (+)<br />
Subtotal (95% CI)<br />
Total events: 235 (infliximab), 102 (placebo)<br />
Test for heterogeneity: 2 = 0.07, df = 1 (p = 0.79), I2 199/343 87/341<br />
36/86 15/88<br />
429 429<br />
= 0%<br />
Test for overall effect: z = 8.63 (p < 0.00001)<br />
02 With concurrent, newly initiated MTX (infliximab + MTX vs MTX)<br />
Quinn, 2005, 141 [54 weeks] (+)<br />
ASPIRE, 135 [54 weeks] (+)<br />
Subtotal (95% CI)<br />
Total events: 227 (infliximab), 153 (placebo)<br />
Test for heterogeneity: 2 = 0.19, df = 1 (p = 0.66), I2 8/10 6/10<br />
219/351 147/274<br />
361 284<br />
= 0%<br />
Test for overall effect: z = 2.29 (p = 0.02)<br />
Total (95% CI)<br />
Total events: 462 (infliximab), 255 (placebo)<br />
Test for heterogeneity: 2 = 35.12, df = 3 (p < 0.00001), I2 790 713<br />
= 91.5%<br />
Test for overall effect: z = 8.20 (p < 0.00001)<br />
FIGURE 35 ACR20 RR: infliximab licensed dose versus placebo (with concurrent MTX)<br />
RR (fixed)<br />
95% CI<br />
0.1 0.2 0.5 1 2 5 10<br />
Favours placebo Favours infliximab<br />
Weight<br />
%<br />
31.94<br />
5.43<br />
37.37<br />
2.20<br />
60.44<br />
62.63<br />
100.00<br />
RR (fixed)<br />
95% CI<br />
2.27 (1.86 to 2.78)<br />
2.46 (1.45 to 4.15)<br />
2.30 (1.90 to 2.78)<br />
1.33 (0.74 to 2.41)<br />
1.16 (1.01 to 1.33)<br />
1.17 (1.02 to 1.34)