A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
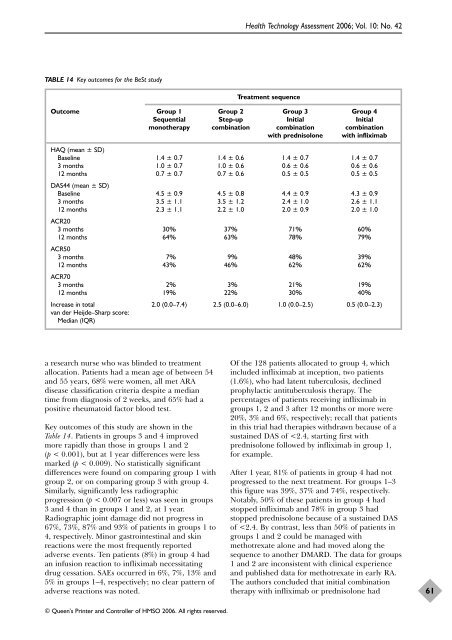
TABLE 14 Key outcomes for <strong>the</strong> BeSt study<br />
a research nurse who was blinded to treatment<br />
allocation. Patients had a mean age <strong>of</strong> between 54<br />
and 55 years, 68% were women, all met ARA<br />
disease classification criteria despite a median<br />
time from diagnosis <strong>of</strong> 2 weeks, and 65% had a<br />
positive rheumatoid factor blood test.<br />
Key outcomes <strong>of</strong> this study are shown in <strong>the</strong><br />
Table 14. Patients in groups 3 and 4 improved<br />
more rapidly than those in groups 1 and 2<br />
(p < 0.001), but at 1 year differences were less<br />
marked (p < 0.009). No statistically significant<br />
differences were found on comparing group 1 with<br />
group 2, or on comparing group 3 with group 4.<br />
Similarly, significantly less radiographic<br />
progression (p < 0.007 or less) was seen in groups<br />
3 and 4 than in groups 1 and 2, at 1 year.<br />
Radiographic joint damage did not progress in<br />
67%, 73%, 87% and 93% <strong>of</strong> patients in groups 1 to<br />
4, respectively. Minor gastrointestinal and skin<br />
reactions were <strong>the</strong> most frequently reported<br />
adverse events. Ten patients (8%) in group 4 had<br />
an infusion reaction to infliximab necessitating<br />
drug cessation. SAEs occurred in 6%, 7%, 13% and<br />
5% in groups 1–4, respectively; no clear pattern <strong>of</strong><br />
adverse reactions was noted.<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
Treatment sequence<br />
Outcome Group 1 Group 2 Group 3 Group 4<br />
Sequential Step-up Initial Initial<br />
mono<strong>the</strong>rapy combination combination combination<br />
with prednisolone with infliximab<br />
HAQ (mean ± SD)<br />
Baseline 1.4 ± 0.7 1.4 ± 0.6 1.4 ± 0.7 1.4 ± 0.7<br />
3 months 1.0 ± 0.7 1.0 ± 0.6 0.6 ± 0.6 0.6 ± 0.6<br />
12 months<br />
DAS44 (mean ± SD)<br />
0.7 ± 0.7 0.7 ± 0.6 0.5 ± 0.5 0.5 ± 0.5<br />
Baseline 4.5 ± 0.9 4.5 ± 0.8 4.4 ± 0.9 4.3 ± 0.9<br />
3 months 3.5 ± 1.1 3.5 ± 1.2 2.4 ± 1.0 2.6 ± 1.1<br />
12 months<br />
ACR20<br />
2.3 ± 1.1 2.2 ± 1.0 2.0 ± 0.9 2.0 ± 1.0<br />
3 months 30% 37% 71% 60%<br />
12 months<br />
ACR50<br />
64% 63% 78% 79%<br />
3 months 7% 9% 48% 39%<br />
12 months<br />
ACR70<br />
43% 46% 62% 62%<br />
3 months 2% 3% 21% 19%<br />
12 months 19% 22% 30% 40%<br />
Increase in total 2.0 (0.0–7.4) 2.5 (0.0–6.0) 1.0 (0.0–2.5) 0.5 (0.0–2.3)<br />
van der Heijde–Sharp score:<br />
Median (IQR)<br />
Of <strong>the</strong> 128 patients allocated to group 4, which<br />
included infliximab at inception, two patients<br />
(1.6%), who had latent tuberculosis, declined<br />
prophylactic antituberculosis <strong>the</strong>rapy. The<br />
percentages <strong>of</strong> patients receiving infliximab in<br />
groups 1, 2 and 3 after 12 months or more were<br />
20%, 3% and 6%, respectively; recall that patients<br />
in this trial had <strong>the</strong>rapies withdrawn because <strong>of</strong> a<br />
sustained DAS <strong>of</strong>