A systematic review of the effectiveness of adalimumab
A systematic review of the effectiveness of adalimumab A systematic review of the effectiveness of adalimumab
54 Effectiveness TABLE 11 Summary of 2-year results from TEMPO study: combination of etanercept (25 mg s.c. twice weekly) plus MTX versus MTX alone in MTX-naïve patients/responders Comparison or outcome N included Statistical method Effect size (95% CI) in analysis ACR20 responder 459 RR (fixed) 1.49 (1.25 to 1.77)* ACR50 responder 459 RR (fixed) 1.92 (1.52 to 2.41)* ACR70 responder 459 RR (fixed) 2.53 (1.82 to 3.54)* RD ACR20 responder 459 RD (fixed) 0.22 (0.13 to 0.30)* RD ACR50 responder 459 RD (fixed) 0.27 (0.19 to 0.36)* RD ACR70 responder 459 RD (fixed) 0.25 (0.17 to 0.33)* SJC, end of study result 459 WMD (fixed) –3.70 (–5.71 to –1.69)* Patients’ global assessment, end of study result 459 WMD (fixed) –1.20 (–1.51 to –0.89)* HAQ, end of study result 459 WMD (fixed) –0.40 (–0.52 to –0.28)* DAS, end of study result Modified van de Heijde–Sharp score, 459 WMD (fixed) –0.80 (–1.02 to –0.58)* mean change from baseline (1-year result) 430 WMD (fixed) –3.34 (–5.12 to –1.56)* Withdrawal for any reasons 459 RR (fixed) 0.61 (0.48 to 0.77)* Withdrawal due to lack of efficacy 459 RR (fixed) 0.27 (0.13 to 0.55)* Withdrawal due to adverse events 459 RR (fixed) 0.80 (0.55 to 1.17) Death 459 RR (fixed) 0.99 (0.06 to 15.68) SAEs 459 RR (fixed) 1.25 (0.87 to 1.81) Malignancy: all 459 RR (fixed) 2.47 (0.48 to 12.59) Malignancy: skin cancer excluding melanoma Malignancy: all cancer excluding 459 RR (fixed) 1.97 (0.18 to 21.62) non-melanoma skin cancer 459 RR (fixed) 2.96 (0.31 to 28.26) Serious infection 459 RR (fixed) 0.86 (0.42 to 1.76) Any infection 459 RR (fixed) 1.00 (0.91 to 1.11) * Statistically significant result at (p < 0.05) least six swollen joints and six tender joints were recruited and followed for 14 weeks. The primary end-point was not stated, although various disease activity measures and serum matrix metalloproteinase-3 (MMP-3) were evaluated. Methods of randomisation, allocation concealment, patient withdrawals and use of ITT analysis were not clearly described. START: Westhovens and colleagues, 2006 105,111 This double-blind, multicentre safety trial compared infliximab, at two doses (3 or 10 mg kg –1 , i.v., at week 0, 2 and 6, then every 8 weeks thereafter), and placebo in patients receiving concurrent methotrexate. Patients were treated for 46 weeks, but patients in the placebo group were switched to receive infliximab 3mgkg –1 every 8 weeks at week 22. Thus results beyond week 22 are excluded from this review and the 22-week results are referred to as the end of trial results. Patients who were receiving methotrexate for at least 3 months and at a stable dose (≤ 25 mg per week) for at least 4 weeks, with a minimum of six swollen joints and six tender joints were recruited. Concomitant stable doses of other DMARDs were allowed. Twenty-five per cent of the patients were receiving one or more DMARDs in addition to methotrexate. The primary end-point was any occurrence of a serious infection within the first 22 weeks after initiating therapy. Quinn and colleagues, 2005 141 This small, double-blind, single-centre RCT compared methotrexate alone (started at 7.5 mg
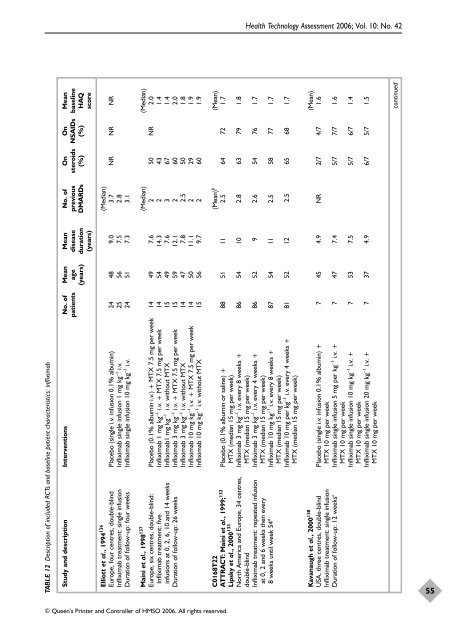
TABLE 12 Description of included RCTs and baseline patient characteristics: infliximab Study and description Interventions No. of Mean Mean No. of On On Mean patients age disease previous steroids NSAIDs baseline (years) duration DMARDs (%) (%) HAQ (years) score Elliott et al., 1994136 (Median) Europe, four centres, double-blind Placebo (single i.v. infusion 0.1% albumin) 24 48 9.0 3.7 NR NR NR Infliximab treatment: single infusion Infliximab single infusion 1 mg kg –1 i.v. 25 56 7.5 2.8 Duration of follow-up: four weeks Infliximab single infusion 10 mg kg –1 i.v. 24 51 7.3 3.1 Maini et al., 1998137 (Median) (Median) Europe, six centres, double-blind: Placebo (0.1% albumin i.v.) + MTX 7.5 mg per week 14 49 7.6 2 50 NR 2.0 Infliximab treatment: five Infliximab 1 mg kg –1 i.v. + MTX 7.5 mg per week 14 54 14.3 2 43 1.4 infusions at 0, 2, 6, 10 and 14 weeks Infliximab1 mg kg –1 i.v. without MTX 15 49 7.6 3 67 1.4 Duration of follow-up: 26 weeks Infliximab 3 mg kg –1 i.v. + MTX 7.5 mg per week 15 59 12.1 2 60 2.0 Infliximab 3 mg kg –1 i.v. without MTX 14 47 7.8 2.5 50 1.8 Infliximab 10 mg kg –1 i.v. + MTX 7.5 mg per week 14 50 11.1 2 29 1.9 Infliximab 10 mg kg –1 i.v. without MTX 15 56 9.7 2 60 1.9 © Queen’s Printer and Controller of HMSO 2006. All rights reserved. Health Technology Assessment 2006; Vol. 10: No. 42 C0168T22 (Mean) b (Mean) ATTRACT: Maini et al., 1999; 132 Placebo (0.1% albumin or saline) + 88 51 11 2.5 64 72 1.7 Lipsky et al., 2000 133 MTX (median 15 mg per week) North America and Europe, 34 centres, Infliximab 3 mg kg –1 i.v. every 8 weeks + 86 54 10 2.8 63 79 1.8 double-blind MTX (median 15 mg per week) Infliximab treatment: repeated infusion Infliximab 3 mg kg –1 i.v. every 4 weeks + 86 52 9 2.6 54 76 1.7 at 0, 2 and 6 weeks then every MTX (median 15 mg per week) 8 weeks until week 54 a Infliximab 10 mg kg –1 i.v. every 8 weeks + 87 54 11 2.5 58 77 1.7 MTX (median 15 mg per week) Infliximab 10 mg per kg –1 i.v. every 4 weeks + 81 52 12 2.5 65 68 1.7 MTX (median 15 mg per week) Kavanaugh et al., 2000 138 (Mean) USA, three centres, double-blind Placebo (single i.v. infusion 0.1% albumin) + 7 45 4.9 NR 2/7 4/7 1.6 Infliximab treatment: single infusion MTX 10 mg per week Duration of follow-up: 12 weeks c Infliximab single infusion 5 mg per kg –1 i.v. + 7 47 7.4 5/7 7/7 1.6 MTX 10 mg per week Infliximab single infusion 10 mg kg –1 i.v. + 7 53 7.5 5/7 6/7 1.4 MTX 10 mg per week Infliximab single infusion 20 mg kg –1 i.v. + 7 37 4.9 6/7 5/7 1.5 MTX 10 mg per week continued 55
- Page 19 and 20: Summary RA is a common, chronic, in
- Page 21 and 22: (IL-2) and interleukin-6 (IL-6), pr
- Page 23 and 24: Assessment of response to DMARDs Re
- Page 25 and 26: combination with methotrexate or al
- Page 27: disease between clinic appointments
- Page 30 and 31: 14 Effectiveness in juvenile arthri
- Page 32 and 33: 16 Effectiveness adalimumab plus me
- Page 34 and 35: 18 Effectiveness Monoclonal Antibod
- Page 36 and 37: 20 TABLE 1 Description of included
- Page 38 and 39: 22 TABLE 2 Quality of included RCTs
- Page 40 and 41: 24 Effectiveness change in modified
- Page 42 and 43: 26 Effectiveness TABLE 4 Meta-analy
- Page 44 and 45: 28 Effectiveness Review: Adalimumab
- Page 46 and 47: 30 Effectiveness Review: Adalimumab
- Page 48 and 49: 32 TABLE 6 Effectiveness Descriptio
- Page 50 and 51: 34 TABLE 6 Effectiveness Descriptio
- Page 52 and 53: 36 TABLE 7 Effectiveness Quality of
- Page 54 and 55: 38 Effectiveness etanercept trials.
- Page 56 and 57: 40 Effectiveness TABLE 9 Summary of
- Page 58 and 59: 42 Effectiveness Review: Etanercept
- Page 60 and 61: 44 Effectiveness Review: Etanercept
- Page 62 and 63: 46 Effectiveness Review: Etanercept
- Page 64 and 65: 48 Effectiveness Review: Etanercept
- Page 66 and 67: 50 Effectiveness Review: Etanercept
- Page 68 and 69: 52 Effectiveness Review: Etanercept
- Page 72 and 73: 56 TABLE 12 Description of included
- Page 74 and 75: 58 TABLE 13 Quality of included RCT
- Page 76 and 77: 60 Effectiveness per week and escal
- Page 78 and 79: 62 Effectiveness significant advant
- Page 80 and 81: 64 Effectiveness Review: Infliximab
- Page 82 and 83: 66 Effectiveness Review: Infliximab
- Page 84 and 85: 68 Effectiveness FIGURE 44 Malignan
- Page 86 and 87: 70 TABLE 17 Effectiveness Summary o
- Page 89 and 90: Summary of review of existing econo
- Page 91 and 92: TABLE 20 Summary of published ICERs
- Page 93 and 94: TABLE 21 Published etanercept econo
- Page 95 and 96: TABLE 23 Published economic analyse
- Page 97 and 98: TABLE 25 Treatment sequences: adali
- Page 99 and 100: management of RA, i.e. 1st and 2nd
- Page 101 and 102: To estimate the long-term consequen
- Page 103 and 104: TABLE 32 TNF inhibitors as last act
- Page 105 and 106: TABLE 34 Basic structure of the mod
- Page 107 and 108: een quit on grounds of toxicity, ad
- Page 109 and 110: TABLE 39 Strategy set: adalimumab a
- Page 111 and 112: TABLE 43 Beta distributions for HAQ
- Page 113 and 114: TABLE 44 Early cessation of DMARDs:
- Page 115 and 116: TABLE 46 Unit costs for tests and v
- Page 117 and 118: following properties, according to
- Page 119 and 120: TABLE 52 Base case: TNF inhibitors
TABLE 12 Description <strong>of</strong> included RCTs and baseline patient characteristics: infliximab<br />
Study and description Interventions No. <strong>of</strong> Mean Mean No. <strong>of</strong> On On Mean<br />
patients age disease previous steroids NSAIDs baseline<br />
(years) duration DMARDs (%) (%) HAQ<br />
(years) score<br />
Elliott et al., 1994136 (Median)<br />
Europe, four centres, double-blind Placebo (single i.v. infusion 0.1% albumin) 24 48 9.0 3.7 NR NR NR<br />
Infliximab treatment: single infusion Infliximab single infusion 1 mg kg –1 i.v. 25 56 7.5 2.8<br />
Duration <strong>of</strong> follow-up: four weeks Infliximab single infusion 10 mg kg –1 i.v. 24 51 7.3 3.1<br />
Maini et al., 1998137 (Median) (Median)<br />
Europe, six centres, double-blind: Placebo (0.1% albumin i.v.) + MTX 7.5 mg per week 14 49 7.6 2 50 NR 2.0<br />
Infliximab treatment: five Infliximab 1 mg kg –1 i.v. + MTX 7.5 mg per week 14 54 14.3 2 43 1.4<br />
infusions at 0, 2, 6, 10 and 14 weeks Infliximab1 mg kg –1 i.v. without MTX 15 49 7.6 3 67 1.4<br />
Duration <strong>of</strong> follow-up: 26 weeks Infliximab 3 mg kg –1 i.v. + MTX 7.5 mg per week 15 59 12.1 2 60 2.0<br />
Infliximab 3 mg kg –1 i.v. without MTX 14 47 7.8 2.5 50 1.8<br />
Infliximab 10 mg kg –1 i.v. + MTX 7.5 mg per week 14 50 11.1 2 29 1.9<br />
Infliximab 10 mg kg –1 i.v. without MTX 15 56 9.7 2 60 1.9<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2006. All rights reserved.<br />
Health Technology Assessment 2006; Vol. 10: No. 42<br />
C0168T22 (Mean) b<br />
(Mean)<br />
ATTRACT: Maini et al., 1999; 132<br />
Placebo (0.1% albumin or saline) + 88 51 11 2.5 64 72 1.7<br />
Lipsky et al., 2000 133<br />
MTX (median 15 mg per week)<br />
North America and Europe, 34 centres, Infliximab 3 mg kg –1 i.v. every 8 weeks + 86 54 10 2.8 63 79 1.8<br />
double-blind MTX (median 15 mg per week)<br />
Infliximab treatment: repeated infusion Infliximab 3 mg kg –1 i.v. every 4 weeks + 86 52 9 2.6 54 76 1.7<br />
at 0, 2 and 6 weeks <strong>the</strong>n every MTX (median 15 mg per week)<br />
8 weeks until week 54 a<br />
Infliximab 10 mg kg –1 i.v. every 8 weeks + 87 54 11 2.5 58 77 1.7<br />
MTX (median 15 mg per week)<br />
Infliximab 10 mg per kg –1 i.v. every 4 weeks + 81 52 12 2.5 65 68 1.7<br />
MTX (median 15 mg per week)<br />
Kavanaugh et al., 2000 138<br />
(Mean)<br />
USA, three centres, double-blind Placebo (single i.v. infusion 0.1% albumin) + 7 45 4.9 NR 2/7 4/7 1.6<br />
Infliximab treatment: single infusion MTX 10 mg per week<br />
Duration <strong>of</strong> follow-up: 12 weeks c<br />
Infliximab single infusion 5 mg per kg –1 i.v. + 7 47 7.4 5/7 7/7 1.6<br />
MTX 10 mg per week<br />
Infliximab single infusion 10 mg kg –1 i.v. + 7 53 7.5 5/7 6/7 1.4<br />
MTX 10 mg per week<br />
Infliximab single infusion 20 mg kg –1 i.v. + 7 37 4.9 6/7 5/7 1.5<br />
MTX 10 mg per week<br />
continued<br />
55